This research found that:
- An epigenetic mechanism underlies the powerful cocaine–environment associations that promote relapse.
- The mechanism may be instrumental in all drug reward–based learning.
A NIDA-sponsored study sheds light on how cocaine creates the powerful drug–environment associations that underlie cue-induced drug seeking and relapse. Researchers found that cocaine blocks an epigenetic process which limits the strength of synapses that link rewarding experiences with associated environments. The new findings suggest that a gene regulator called histone deacetylase 5 (HDAC5) may offer new opportunities for reducing relapse risk.
Cocaine and HDAC5
Drs. Makoto Taniguchi and Christopher Cowan from the Medical University of South Carolina and Harvard Medical School and colleagues have been investigating HDAC5 and its role in responses to cocaine. HDAC5 is one of a group of enzymes that enhance or reduce transcription, and hence expression, of their target genes. The enzyme is found both inside and outside the cell nucleus, but only has access to its target genes when it is inside.
The researchers previously showed that cocaine causes HDAC5 molecules in the nucleus accumbens (NAc) to pick up phosphate groups that lock it out of the cell nucleus. They now report that excluding HDAC5 from NAc nuclei bolsters cocaine–environment associations and cue-induced responses to the drug. The researchers exposed two groups of mice with different levels of NAc nuclear HDAC5 to cocaine in a test chamber. When they later gave the animals the option of returning to the chamber in which they had received the drug or another chamber, the group with less NAc nuclear HDAC5 was more likely to prefer the chamber associated with exposure. These mice also exhibited more cue-induced relapse-like behavior when re-exposed to the cocaine-associated test chamber after a period without access to the drug.
HDAC5 and Its Target Gene
Further experiments revealed the mechanism by which cocaine’s exclusion of HDAC5 from NAc nuclei strengthens drug–environment associations.
First, Drs. Taniguchi and Cowan and colleagues conducted a screen to identify HDAC5's target genes. Among the many genes that HDAC5 affected, one stood out. Npas4 encodes a protein, NPAS4, that promotes the strengthening of neural connections.
The researchers demonstrated that when HDAC5 interacts with Npas4, it reduces expression of the gene. Conversely, when cocaine prevents HDAC5 from entering the nucleus and interacting with Npas4, the gene is robustly expressed (see Figure). Thus, administering cocaine to mice or placing the animals in a test chamber where they previously received the drug resulted in a rapid increase in Npas4 expression.
The researchers next linked the cocaine-induced increase in Npas4 expression to the formation of strong drug–environment associations. In these experiments, blocking Npas4 expression in the NAc of mice weakened the animals’ attraction to a chamber in which they had received the drug and slowed their acquisition of other drug-reinforced actions.
Drs. Taniguchi and Cowan and colleagues concluded that cocaine’s effects on HDAC5 and Npas4 are keys to the powerful drug–environment associations that drive cue-induced drug seeking and relapse. By excluding HDAC5 from cell nuclei, the drug removes a brake on Npas4 expression in the NAc. NPAS4 levels rise and enhance synaptic changes that are presumed to be the physical basis for the associations. The researchers suggest that this same NAc mechanism may be instrumental in all learning that involves linking drug reward experiences and their associated circumstances in memory.
"Nuclear shuttling of HDAC5 is an unexpected and exciting neuroregulatory mechanism," says Dr. John Satterlee from the Division of Neuroscience and Behavior in NIDA's Genetics, Epigenetics, and Developmental Neuroscience Branch. "In the long term, this foundational knowledge could even lead to the development of a novel therapeutic to treat cocaine use disorder."
This study was supported by NIH grants DA027664, DA032708, DA10460, DA003906, DA007288, and DA036319.
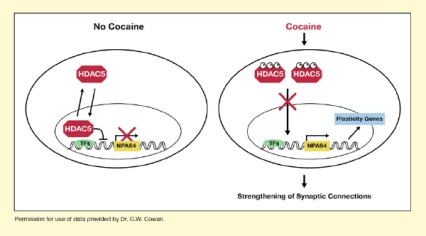
- Text Description of Figure
-
The figure illustrates how cocaine affects the actions of HDAC5 and how this results in the strengthening of synaptic connections. Both parts of the figure show a cell in the nucleus accumbens, represented by a large oval, with a smaller oval representing the nucleus inside the cell. In the nucleus, a piece of DNA is shown, represented by a double wave line. A green oval represents a transcription factor bound to the DNA, and a yellow rectangle represents the NPAS4 gene. Red hexagons represent HDAC5 molecules. The left half of the figure shows the actions of HDAC5 in the absence of cocaine. HDAC5 molecules can move into and out of the cell nucleus as illustrated by two black arrows. In the nucleus, HDAC5 interacts with the transcription factor. A curved black line reaching from HDAC5 to the DNA indicates that this interaction blocks transcription of NPAS4. A red cross over the black arrow above the yellow NPAS4 rectangle indicates that no NPAS4 transcription takes place.
The right side of the figure shows the actions of HDAC5 in the presence of cocaine. The red HDAC5 molecules each have three white circles with a "P" inside, indicating phosphorylation. A black downward arrow with a red cross over it indicates that the phosphorylated HDAC5 molecules can no longer enter the cell nucleus. The green transcription factor allows transcription of the yellow NPAS4 gene, indicated by the black arrow above the gene. A black arrow on the right indicates that this leads to the activation of plasticity genes, represented by a light blue rectangle. This process leads to the strengthening of synaptic connections.
Source:
- Taniguchi, M., Carreira, M.B., Cooper, Y.A., et al. HDAC5 and its target gene, Npas4, function in the nucleus accumbens to regulate cocaine-conditioned behaviors. Neuron 96(1):130-144.e6, 2017.