This research found that:
- Cocaine produces a portion of its rewarding effects by increasing levels of granulocyte-colony stimulating factor (G-CSF) in the brain’s reward center.
- Treatments that prevent G-CSF signaling in the nucleus accumbens (NAc) might reduce motivation to use cocaine.
Dr. Erin Calipari, Dr. Drew Kiraly, and colleagues from the Icahn School of Medicine at Mount Sinai in New York have identified granulocyte-colony stimulating factor (G-CSF) as a potential target for medications to treat cocaine use disorder. Their work suggests that medications which modulate G-CSF levels could reduce motivation to take the stimulant without affecting desire for healthy rewards. Moreover, in their experiments, G-CSF produced no potentially addictive effects of its own.
G-CSF is a protein that is secreted by immune and other cells throughout the body and binds to cell-surface receptors to produce a wide variety of effects. The Mount Sinai researchers administered G-CSF followed by cocaine to a group of mice, and cocaine alone to a second group. They exposed the mice to a battery of behavioral tests and observed that the group pretreated with G-CSF exhibited:
- More locomotor movement (i.e., moving from one point to another) compared with the group that only received cocaine, indicating greater sensitivity to cocaine’s stimulant effect
- Stronger preference for places where they had received cocaine, indicating more sensitivity to cocaine’s rewarding effects
- Willingness to expend more effort to receive infusions/injections of cocaine, indicating greater motivation for the drug
- Greater cocaine consumption
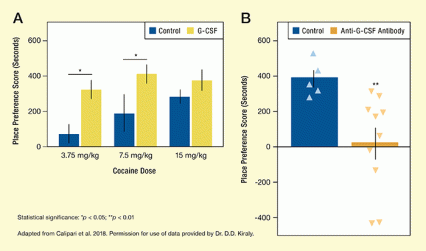
Conversely, lowering G-CSF in the NAc reduced animals’ motivation to seek out places where they had received cocaine. This suggests that when G-CSF activity in the NAc is blocked, cocaine no longer produces rewarding effects (see Figure).
Basis for a Medication?
The Mount Sinai study carried forward previous research indicating that stimulant drugs produce some of their addictive effects by disrupting the immune system. Dr. Kiraly explains, “Over the past 10 years, there has been a growing awareness that changes in immune system function play a role in the pathophysiology of many mental illnesses, but this has not been studied as extensively in addiction.”
The researchers homed in on G-CSF by administering cocaine to mice and observing the effect on serum levels of 32 immune proteins. The only protein to rise significantly and show some indication of a behavioral effect was G-CSF. Shifting to the brain, the researchers found that:
- Cocaine increased G-CSF production in the NAc, a key region in the brain’s reward system.
- G-CSF increased neuronal activation in the NAc, including cells that respond to dopamine. This latter activation may account for the protein’s reward-enhancing effect, as dopamine is a key neurotransmitter in the brain’s reward system.
When the researchers looked at how cocaine increases G-CSF production in the NAc, their experiments pointed to an indirect pathway. Their evidence suggests a positive feed-forward system: Cocaine increases activity of neurons in the medial prefrontal cortex, which increases their excitatory output to the NAc, which prompts NAc neurons to ramp up G-CSF production, which further activates the NAc neurons, ultimately leading to enhanced behavioral effects.
Dr. Kiraly and colleagues propose that regulating G-CSF levels might be an effective treatment strategy for cocaine use disorder. They cite two potential advantages that such a strategy might have over targeting other molecules that have been implicated in cocaine’s effects. In experiments, they found that G-CSF reduced animals’ motivation for cocaine reward without affecting their motivation for other rewards such as sucrose. In addition, G-CSF showed no signs of potential to cause abuse on its own.
Dr. Kiraly says, “Our study raises the possibility that factors outside of the brain can affect the development of addiction, and, even more excitingly, the possibility that we could treat addiction by altering a factor in the blood.” A next step for his team will be to analyze how G-CSF affects mouse behavior in models of drug relapse.
This study was supported by NIH grants DA044308, DA042111, and DA008227.
- Text Description of Figure
-
The two bar charts illustrate that G-CSF in the nucleus accumbens mediates place preference for cocaine in mice. The bar chart on the left compares place preference for control mice treated only with different cocaine doses, indicated in blue, and for mice treated with G-CSF followed by the different cocaine doses, indicated in yellow. They vertical y-axis shows the place preference score calculated as the time spent at in the cocaine-associated chamber minus the time spent in the saline-associated chamber, from 0 to 600 seconds. The two bars on the left indicated control and G-CSF-treated mice that received 3.75 mg/kg cocaine. The place preference score for the control mice is about 70 seconds, and the score for the G-CSF-treated mice is about 320 seconds; a horizontal line and asterisk above the two bars indicate that this difference is statistically significant. The two bars in the middle are for control and G-CSF-treated mice that received 7.5 mg/kg cocaine. The place preference score for the control mice is about 190 seconds, and the score for the G-CSF-treated mice is about 420 seconds; a horizontal line and asterisk above the two bars indicate that this difference is statistically significant. The two bars on the right are for control and G-CSF-treated mice who received 15 mg/kg cocaine. The place preference score for the control mice is about 300 seconds, and the score for the G-CSF-treated mice is about 380 seconds; this difference is not statistically significant.
The bar chart on the right compares place preference for control mice, indicated in blue, and mice that were treated with anti-G-CSF antibody infused into the nucleus accumbens, indicated in orange. Triangles indicate the scores for individual mice. The vertical y-axis shows place preference scores from -400 to 600 seconds. The place preference score for the control mice as a group is about 400 seconds, with the values for individual mice ranging from about 300 seconds to about 450 seconds. The place preference score for the anti-G-CSF-treated mice as a group is about 20 seconds, with values for individual mice ranging from about -400 seconds to about 350 seconds. The two asterisks above the bar for the anti-G-CSF-treated mice indicate that this difference is statistically significant.
Source:
- Calipari, E.S., Godino, A., Peck, E.G., et al. Granulocyte-colony stimulating factor controls neural and behavioral plasticity in response to cocaine. Nature Communications. 2018;9:9. DOI: 10.1038/s41467-017-01881-x.