These studies:
- Identified factors that affect the risk for and severity of neonatal abstinence syndrome (NAS).
- Suggest that reducing expectant mothers’ polysubstance use during buprenorphine treatment for opioid use disorder is an important goal for reducing NAS severity.
Infants who have been exposed to opioids in the womb are at risk of developing symptoms of opioid dependence and withdrawal, known as neonatal abstinence syndrome (NAS), at birth. They may require extended hospital stays and treatment with tapering doses of morphine to resolve their dependence and control symptoms that can include tremors and muscle rigidity, high-pitched and excessive crying, and difficulty breathing and feeding, among others.
A recent NIDA-sponsored study found higher rates of NAS among males than among females. A second study found that, among infants whose mothers were treated with buprenorphine while pregnant, NAS was more severe among those whose mothers used illicit drugs or misused prescription drugs.
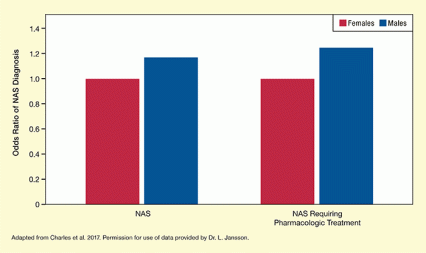
In the largest study of NAS risk to date, Dr. Katherine Charles and colleagues from Vanderbilt University evaluated health records from more than 100,000 mothers and infants who received care from 2009 to 2011 at 108 facilities within Tennessee's Medicaid program. They found that 927 of the infants had been diagnosed with NAS, and boys were 18 percent more likely than girls to receive an NAS diagnosis. Boys also were 24 percent more likely than girls to have NAS that required treatment (see Figure).
Why male infants should be at greater risk of NAS is not clear. Some researchers have suggested that males may be more sensitive than females to the methadone and buprenorphine used to treat their mothers' opioid use disorder (OUD).
In the second study, Dr. Lauren Jansson and colleagues from Johns Hopkins University recorded 41 mothers’ buprenorphine doses and other substance use during pregnancy and assessed their newborns for NAS. Twenty-four of the infants developed NAS symptoms severe enough to require morphine for relief. The researchers' analysis linked maternal polysubstance use to greater infant NAS severity. Infants whose mothers used other substances besides buprenorphine while pregnant required almost 10 times more morphine for NAS than infants whose mothers took only buprenorphine.
Higher maternal doses of buprenorphine also were independently associated with more severe NAS, yet this association became nonsignificant when polysubstance use was added to the model. Mothers who received higher buprenorphine doses also had smaller infants, a finding that remained significant after controlling for polysubstance exposure. Accordingly, the Johns Hopkins researchers suggest that treatment providers consider transitioning pregnant patients to methadone treatment if they continue to crave opioids or relapse while on buprenorphine maintenance therapy, rather than prescribing escalating doses of buprenorphine once a therapeutic dose is reached.
Dr. Jansson notes that a previous study found that buprenorphine is safe and effective for treating opioid dependence during pregnancy and may produce less severe NAS than methadone. Her own findings indicate that, despite that possible advantage, buprenorphine is not the optimal treatment for all women. She says, "Women with OUD who become pregnant should have choices when it comes to medication-assisted treatment. With maternal use of other drugs being the most important driver of more severe NAS, women’s ability to avoid such use is critical. Some may need methadone to alleviate cravings and avoid relapse, stay in treatment, and continue to receive obstetric and psychiatric care. One size does not fit all." For pregnant women who do receive buprenorphine, she adds, treatment should optimally be given in comprehensive treatment settings and include strong psychosocial components to address other substance use and misuse to optimize the outcome of the infant.
Dr. Jansson's study evaluated buprenorphine monotherapy. Her group is currently testing the effects of combination therapy with buprenorphine and naloxone on mothers and infants. She says, "This research will provide us with information that we can use to inform providers and patients about the effects of these medications, and to provide the best possible medication for each pregnant woman who seeks treatment for opioid use disorder."
These studies were supported by NIH grants DA038720 and DA031689.
- Text Description of Figure
-
The bar chart shows the different odds ratios of NAS and NAS requiring pharmacologic treatment in female and male infants. Red bars indicate female infants, blue bars represent male infants. The vertical y-axis represents the odds ratio on a scale from 0 to 1.4. The two bars on the left show the odds ratios of a NAS diagnosis. The odds ratio for female infants has been set at 1.0; the odds ratio for male infants is 1.18. The two bars on the right show the odds ratios of a diagnosis of NAS requiring pharmacological treatment. The odds ratio for female infants has again been set at 1.0; the odds ratio for males is 1.24.
Sources:
- Charles, M.K., Cooper, W.O., Jansson, L.M., et al. Male sex associated with increased risk of neonatal abstinence syndrome. Hosp Pediatr 7(6):328-334, 2017.
- Jansson, L.M., Velez, M.L., McConnell, K., et al. Maternal buprenorphine treatment and infant outcome. Drug Alcohol Depend 180:56-61, 2017.