This research:
- Traced the effects of cocaine-induced disruption of serotonin regulation in the ventral pallidum and orbitofrontal cortex.
- Suggests that these effects may contribute to drug-seeking and cocaine-associated cognitive impairments.
Cocaine causes addiction and cognitive deficits, resulting in uncontrolled use of the drug and impaired decision making. New NIDA-supported research suggests that the drug may produce these problems in part by disrupting regulation of the neurotransmitter serotonin.
Cocaine blocks the transporters for dopamine, serotonin, and norepinephrine, causing increases in extracellular levels of these neurotransmitters. Sharp spikes in extracellular dopamine, particularly in the nucleus accumbens (NAc) reward center, account for much of the drug's reinforcing and addicting effects. The new studies suggest that increased extracellular serotonin in the ventral pallidum (VP) and orbitofrontal cortex (OFC) also may contribute to reward and may underlie cognitive deficits observed in users of the drug.
The VP: Reward and Craving
Dr. Aya Matsui of the Intramural Research Program (IRP) of the National Institute on Alcohol Abuse and Alcoholism and Dr. Veronica Alvarez of NIDA's IRP investigated cocaine-induced alteration of serotonin in the VP. Their findings suggest that cocaine-induced increases in serotonin in the VP could weaken one’s ability to curb impulsive behaviors.
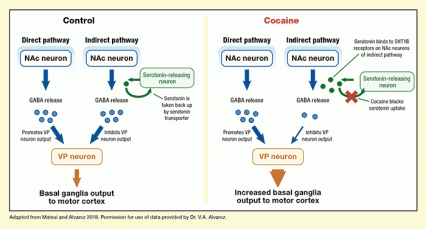
The VP participates in a circuit in the basal ganglia that converts the anticipation of rewards into action. It receives gamma aminobutyric acid (GABA) neurotransmitter input from the NAc reward center via two distinct pathways and relays it forward, ultimately to the motor cortex. GABA transmission via the "direct" pathway excites basal ganglia output; via the "indirect" pathway, it inhibits basal ganglia output. The relative strength of GABA transmission in the two pathways determines whether neurons in the motor cortex will be sufficiently excited to initiate movement.
Dr. Alvarez and Dr. Matsui exposed mouse VP tissue to cocaine and tracked the effects on serotonin and GABA with electrophysiology, voltammetry, and immunofluorescence techniques. The cocaine-induced increase in serotonin levels reduces NAc GABA neurotransmission to the VP via the indirect pathway, but not the direct pathway (see Figure). As the direct pathway was unaffected while the indirect was reduced, the relative influence of the direct pathway increased.
The IRP researchers surmise that the cocaine-induced increase in the influence of the direct pathway might amplify the drug's rewarding effects. As well, it could prime motor cortex neurons to respond more readily to drug craving and thereby increase the difficulty of resisting craving.
The OFC: Cognitive Flexibility
Dr. Andrew Wright, a graduate student in the NIH Graduate Partnership Program with Brown University; Dr. Carl Lupica; and colleagues at NIDA’s IRP measured serotonin’s effects on neuronal activity in tissue from the OFC of drug-free rats and rats that had been exposed to cocaine for 12 days and then maintained without the drug for 11 to 73 days. They found that treating OFC tissue with serotonin overall inhibited neuronal activity in the drug-free animals but increased it in those with a history of cocaine exposure and withdrawal. The difference reflected changes in how two serotonin receptors, 5-HT1A and 5-HT1B, responded to the neurotransmitter in the drug-free versus the exposed and withdrawn animals.
Neurons in the OFC monitor the many alternative courses of action available to an animal or a person at each moment, together with estimates of their potential risks and rewards. A well-functioning OFC helps one identify and select actions that advance one’s primary goals. Disruption here can impair one’s ability to learn from experience and adjust one's behavior to fit changing circumstances. Based on the new findings, Dr. Lupica says, "The lack of cognitive flexibility and impaired reasoning seen in people addicted to cocaine may result from long-term disruption of serotonin system function."
Potential Pharmacotherapies for Cocaine Use Disorder?
The two studies indicate that cocaine exerts its reinforcing effects and causes cognitive impairment not only by interfering with dopamine regulation but also by altering serotonin regulation in the brain. Both research teams hope that their findings can inform future research into pharmacotherapies for cocaine use disorder and believe that effective treatments should target multiple neuronal pathways.
Dr. Alvarez says, "We reason that an effective therapy for cocaine addiction should take into account the serotonin actions of cocaine. Treatments will have to account for the inhibition of GABA transmission, which is mediated by dopamine in the nucleus accumbens and by serotonin in the ventral pallidum."
- Text Description of Figure
The figure illustrates how cocaine affects serotonin signaling in the NAc and VP and the resulting effects. The left panel shows NAc-to-VP signaling under control conditions. There are two signaling pathways from the NAc to the VP; the left part of the panel shows the direct pathway and the right part of the panel shows the indirect pathway. Blue rectangles near the top represent NAc neurons, which release GABA, indicated by blue circles. GABA release by NAc neurons in the direct pathway promotes VP neuron output, whereas GABA release by NAc neurons in the indirect pathway reduces VP neuron output as shown by the two blue arrows. The brown rectangle near the bottom represents the VP neuron, and the brown arrow pointing down indicates the strength of the basal ganglia output to the motor cortex. The green rectangle in the upper right part of the panel represents a serotonin-releasing neuron, with released serotonin represented by a green circle. As indicated by the green arrow, the serotonin is directly taken back up into the serotonin-releasing neuron, resulting in only low levels of free serotonin indicated by only one green circle.
The right panel of the figure shows NAc-to-VP signaling in the presence of cocaine. The blue rectangles near the top again represent the NAc neurons of the direct pathway on the left and the indirect pathways on the right, and the green rectangle again represents a serotonin-releasing neuron. As indicated by a red cross over the green arrow, cocaine prevents reuptake of serotonin into the serotonin-releasing neuron, resulting in increased levels of free serotonin, indicated by four green circles. Serotonin can interact with 5-HT1B receptors on NAc neurons of the indirect pathway. This reduces GABA release by these neurons, as indicated by the smaller number of blue circles, but not by the neurons of the direct pathway. Fewer GABA molecules in the indirect pathway lead to reduced inhibition of VP neuron output as indicated by the smaller blue arrow, whereas GABA release and actions in the direct pathway are not affected. As a result, overall output from the VP neuron indicated in brown to the motor cortex is increased, as shown by a wider brown arrow.
Sources:
- Matsui, A. and Alvarez, V.A. Cocaine inhibition of synaptic transmission in the ventral pallidum is pathway-specific and mediated by serotonin. Cell Reports 23(13):3852-3863, 2018.
- Wright, A.M., Zapata, A., Baumann, M.H., et al. Enduring loss of serotonergic control of orbitofrontal cortex function following contingent and noncontingent cocaine exposure. Cerebral Cortex 27(12):5463-5476, 2017.