This study reported:
- Interim treatment with buprenorphine significantly improved the psychiatric symptoms of people awaiting comprehensive treatment for opioid use disorder (OUD).
- Buprenorphine treatment, even without concurrent psychosocial counseling, may help patients with no, or delayed, access to comprehensive OUD treatment.
The prevalence of opioid use disorder in many parts of the country outstrips the capacity of treatment programs, and many who seek help must wait to be admitted, sometimes for lengthy periods. NIDA-sponsored researchers recently reported that providing waitlisted patients with technology-supported buprenorphine helped them reduce their illicit opioid use during the wait time. The researchers have now found that the treatment also significantly reduced the patients’ psychiatric symptoms, even though no counseling was provided.
Ms. Joanna Streck, Dr. Stacey Sigmon, and colleagues at the University of Vermont developed and tested the interim treatment in a 12-week study. After a 1-week induction onto buprenorphine, 25 patients received the medication to take at home, with their access and dosage closely controlled by a programmable dispensing device. They also received nightly phone calls via an automated interactive voice response system, which prompted them to report their own opioid use and cravings, encouraged them to attend support groups, and provided urgent access to study staff. No psychosocial counseling was provided as part of the interim dosing regimen.
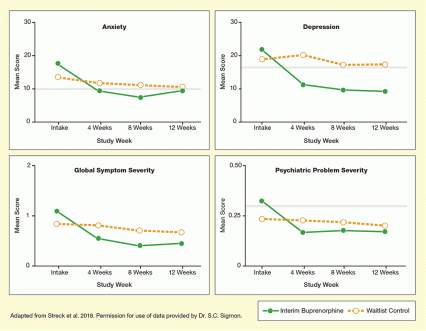
These 25 patients and an equal number in a control group completed standardized assessments for mood and other psychiatric symptoms at study entry and monthly during clinic visits where their urine was tested and progress monitored. At study entry, both groups’ mean scores on the Beck Depression Inventory and Beck Anxiety Inventory were above the cutoff for clinically significant depression and anxiety (see Figure). At each subsequent evaluation, the group receiving buprenorphine reported significantly fewer symptoms compared with study entry, while the control group did not. Similarly, the buprenorphine recipients’ global psychiatric symptoms and scores on the Addiction Severity Index, but not those of the control group, were significantly reduced.
"It is exciting that providing just some basic pharmacotherapy and technology-assisted monitoring may attenuate psychiatric distress in this vulnerable group while they await more comprehensive treatment," says Dr. Sigmon. She notes that the prevalence of psychiatric disorders among people with opioid use disorders is "very high." At their entry into this study, 48 percent of the participants reported psychiatric symptoms at levels indicative of clinically significant disorders.
Dr. Sigmon continues, "That triage buprenorphine dosing mitigates psychological distress as well as reduces opioid use provides further support for considering this approach when access to more comprehensive care is delayed or unavailable." She and her colleagues are now conducting a larger trial of the effects of interim buprenorphine treatment on illicit opioid use and psychiatric symptoms over a longer duration, as well as among patients who live in rural, medically underserved geographic areas.
This study was supported by NIH grants DA042790 and DA037385.
- Text Description of Figure
The figure shows four graphs illustrating changes in psychiatric symptom severity in patients treated with interim buprenorphine or in a waitlist control group over a 12-week study period. Green lines indicate scores for buprenorphine-treated patients and dashed gold lines indicate scores for waitlist control patients.
The top left chart shows anxiety levels in both patient groups. The horizontal x-axis shows the timepoints of measurements (study intake and 4, 8, and 12 weeks of treatment), and the horizontal y-axis shows the mean score on the Beck Anxiety Index from 0 to 30. A gray horizontal line at a mean score of 10 indicates the cut-off for mild anxiety. Scores for the buprenorphine-treated patients were about 18 at intake, about 10 at 4 weeks, about 8 at 8 weeks, and about 10 at 12 weeks. Scores for the waitlist control patients were about 13 at intake, about 12 at 4 weeks, about 11 at 8 weeks, and about 10 at 12 weeks.
The top right chart shows depression levels in both patient groups. The horizontal x-axis shows the timepoints of measurements (study intake and 4, 8, and 12 weeks of treatment), and the horizontal y-axis shows the mean score on the Beck Depression Index from 0 to 30. A gray horizontal line at a mean score of 17 indicates the cut-off for clinically significant depression. Scores for the buprenorphine-treated patients were about 23 at intake, about 11 at 4 weeks, about 10 at 8 weeks, and about 10 at 12 weeks. Scores for the waitlist control patients were about 18 at intake, about 21 at 4 weeks, about 17 at 8 weeks, and about 17 at 12 weeks.
The bottom left chart shows global symptoms severity levels in both patient groups. The horizontal x-axis shows the timepoints of measurements (study intake and 4, 8, and 12 weeks of treatment), and the horizontal y-axis shows the mean score on the Global Severity Index from 0 to 2. Scores for the buprenorphine-treated patients were about 1.1 at intake, about 0.5 at 4 weeks, about 0.4 at 8 weeks, and about 0.5 at 12 weeks. Scores for the waitlist control patients were about 0.8 at intake, about 0.8 at 4 weeks, about 0.7 at 8 weeks, and about 0.6 at 12 weeks.
The bottom right chart shows psychiatric problem severity in both patient groups. The horizontal x-axis shows the timepoints of measurements (study intake and 4, 8, and 12 weeks of treatment), and the horizontal y-axis shows the mean score on the psychiatric composite score on the Addiction Severity index from 0 to 0.5. A gray horizontal line at a mean score of 0.27 indicates the cut-off for problem severity requiring mental health services. Scores for the buprenorphine-treated patients were about 0.28 at intake, about 0.16 at 4 weeks, about 0.16 at 8 weeks, and about 0.16 at 12 weeks. Scores for the waitlist control patients were about 0.24 at intake, about 0.23 at 4 weeks, about 0.21 at 8 weeks, and about 0.20 at 12 weeks.
Source:
- Streck, J.M., Ochalek, T.A., Badger, G.J., et al. Interim buprenorphine treatment during delays to comprehensive treatment: Changes in psychiatric symptoms. Experimental and Clinical Psychopharmacology 26(4):403-409, 2018.