This study found:
- The degree of connectivity of a neuronal network in the rat brain before nicotine exposure can help predict nicotine dependence severity after nicotine exposure as well as reversal of dependence after a period of abstinence.
- Individual differences in connectivity of such circuits may potentially serve as a biomarker for risk of nicotine dependence.
Both in rats and in humans, the brain is organized in a complex network of brain circuits that interact with each other. Within these circuits, certain regions, or modules, have particularly strong internal connections but are sparsely connected to other brain regions. Such modules, as well as the connectivity between and within modules, can be identified by imaging techniques such as functional MRI (fMRI) and may potentially be used as biomarkers of substance use disorder risk.
Dr. Li-Ming Hsu, Dr. Robin J. Keeley, and colleagues from NIDA’s Intramural Research Program and the Chinese Harbin Institute of Technology recently reported that in rats, the strength of connectivity within and between specific brain modules measured before exposure to nicotine could predict whether an individual rat would show signs of nicotine dependence after chronic drug administration. These data from rats may someday help scientists identify circuits in humans that can predict whether some people who experiment with smoking become addicted while others do not. According to the study’s senior investigator, Dr. Elliot Stein, their findings also suggest that the same circuits may predict dependence reversal—or how easily an individual can recover from nicotine dependence.
Linking Rat Brain Circuits and Dependence Severity
To assess whether any such modules were linked to risk of nicotine dependence, animals first underwent an fMRI before nicotine administration began. Next, Dr. Hsu, Dr. Keeley, and colleagues intermittently administered nicotine to naïve rats for 2 weeks to induce nicotine dependence. They then measured dependence severity after the last nicotine administration as well as after 2 weeks of forced abstinence by giving the animals a drug that induced nicotine withdrawal. The more withdrawal behaviors the animals exhibited, the more severe their dependence was considered to be.
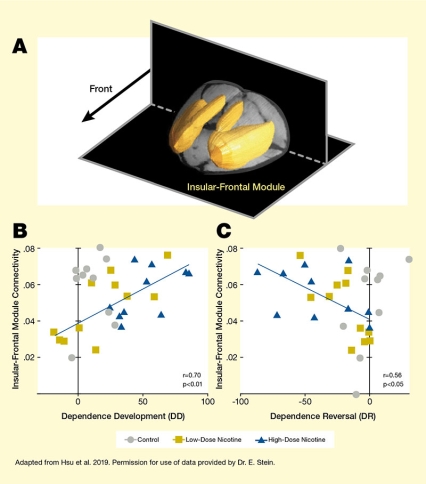
Note: DD = dependence score on Day 15 – dependence score on Day 0; DR = dependence score on Day 29 – dependence score on Day 15. See full text description at end of article
Analysis of the fMRI scans identified five modules (insular-frontal, thalamic, frontal-executive, sensory association, and striatal modules) in the rat brain. The researchers then examined whether the connectivity between or within these modules prior to nicotine administration could be linked to subsequent nicotine dependence and/or dependence reversal after extended abstinence. They found that the functional connectivity strength of the insular-frontal module with the rest of the brain reliably predicted the development of nicotine dependence and reversal (see Figure). Animals with high functional connectivity of this module exhibited more severe dependence but also showed greater dependence reversal after abstinence. Further analysis of the insular-frontal module revealed three sub-modules whose functional-connectivity strength also predicted nicotine dependence. Additionally, two of the three sub-modules predicted dependence reversal.
Do the Findings Apply to Humans?
These results suggest for the first time that pre-existing individual differences in the connections between brain circuits may help predict an individual’s risk for nicotine dependence. Dr. Stein hopes that his team’s preclinical model may someday be applied to the development of nicotine dependence severity in humans. “In about 10 years, we will get our first answers from the longitudinal Adolescent Brain Cognitive Development (ABCD) Study, which will identify children who start smoking as adolescents. Using that information, we can conduct this same type of analysis on their pre-smoking baseline data to see if differences in the circuit we first identified in rodents is indeed predictive of outcomes in humans as well.” If this holds true, brain connectivity in this circuit could serve as a biomarker to identify people most at risk for nicotine dependence or most likely to recover from dependence following treatment.
This study was supported by NIDA’s Intramural Research Program and a grant from the U.S. Food and Drug Administration’s Center for Tobacco Products.
- Text Description of Figure
-
The figure shows the association between connectivity of the insular-frontal module and nicotine dependence development and dependence reversal in rats. (A) The top panel indicates the location of the insular-frontal module in the rat brain. The two black shapes represent horizonal and vertical planes through the rat brain. A black arrow in the top left quadrant points to the front of the rat brain. The yellow structures represent the insular-frontal module, which is located toward the top and front of the brain.
(B) The bottom left graph shows the relationship between insular-frontal module connectivity and dependence development (DD). The horizontal x-axis shows the DD score (measured as a composite score of behavioral responses on Day 15 minus the score on Day 0), on a scale from 0 to 100. The vertical y-axis shows insular-frontal module connectivity on a scale from 0 to 0.08. Gray circles represent data points for control animals; gold squares represent data points from animals treated with low-dose nicotine on Days 1 to 15; and blue triangles represent animals treated with high-dose nicotine on Days 1 to 15. A blue line running from the bottom left to the top right indicates the relationship between increasing connectivity and increasing dependence development. This line crosses the y-axis (no dependence development) at a connectivity score of 0.04 and reaches a maximum with a DD score of about 90 and a connectivity score of about 0.07.
(C) The bottom right graph shows the relationship between insular-frontal module connectivity and dependence reversal (DR). The horizontal x-axis shows the DR score (measured as a composite score of behavioral responses on Day 29 minus the score on Day 15), on a scale from -100 to 0. The vertical y-axis shows insular-frontal module connectivity on a scale from 0 to 0.08. Gray circles represent data points for control animals; gold squares represent data points from animals treated with low-dose nicotine on Days 1 to 15; and blue triangles represent animals treated with high-dose nicotine on Days 1 to 15. A blue line running from the top left to the bottom right indicates the relationship between increasing connectivity and increasing dependence development. This line starts in the top left with a DR score of about -90 and a connectivity score of about 0.07 and crosses the y-axis (no dependence reversal) at a connectivity score of about 0.04.
Source:
- Hsu L.-M., Keeley, R.J., Liang, X., et al. Intrinsic insular-frontal networks predict future nicotine dependence severity. Journal of Neuroscience 39(25): 5028-2037, 2019.