In this study:
- Rats with a disrupted transcription factor 7-like 2 (Tcf7l2) gene in the medial habenula showed markedly greater nicotine intake than control rats.
- Reduced Tcf7l2 expression in the medial habenula reduced the normally observed increase in blood sugar in response to nicotine.
- Rats with decreased Tcf7l2 activity did not experience the diabetes-promoting effects of nicotine.

Nicotine can increase blood sugar levels in humans, and people who smoke have a much higher risk of diabetes than nonsmokers. In a recent NIDA-sponsored study, researchers in the lab of Dr. Paul J. Kenny and colleagues from the Icahn School of Medicine at Mount Sinai and other institutions have shed light on the association between smoking and diabetes. The researchers identified a protein, transcription factor 7-like 2 (Tcf7l2), that is found in high levels in a brain region called the medial habenula and that links nicotine’s addictive properties to its diabetes-promoting actions.
Tcf7l2 Is Part of a Signaling Cascade
Among its many actions, nicotine activates nicotinic cholinergic receptors in the medial habenula; this activation induces unpleasant responses that cause an animal or person to avoid nicotine. The researchers had previously shown that the neuropeptide glucagon-like peptide-1 (GLP-1) enhances habenular function and thereby increases nicotine avoidance behaviors in rats. In the current study, the investigators explored how GLP-1 acts in the habenula to control nicotine intake.
Tcf7l2 is a major component of the signaling cascade that translates GLP-1’s actions into changes in gene expression. The researchers therefore investigated this protein’s role in controlling nicotine intake. Because GLP-1/Tcf7l2 signaling has been implicated in type 2 diabetes, they also analyzed nicotine’s effects on blood glucose regulation and the contribution of Tcf7l2 activity in the medial habenula in this process.
Tcf7l2 Regulates Nicotine Intake
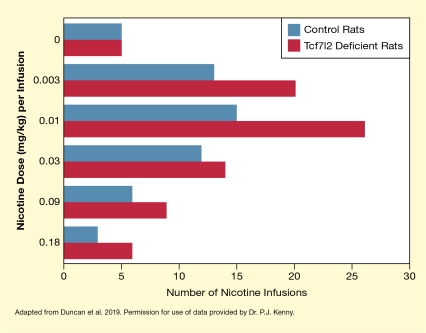
The investigators conducted a series of experiments using rats that had been genetically modified to carry a defective Tcf7l2 gene with reduced activity in the medial habenula. When these animals were trained to self-administer nicotine, they responded more vigorously and consumed greater nicotine quantities than did control rats with an intact Tcf7l2 gene (see Figure 1). Further experiments suggested that the Tcf7l2-deficient rats developed greater tolerance to some of nicotine’s aversive effects that normally limit nicotine consumption.
Tcf7l2 Regulates the Glycemic Response to Nicotine and Nicotine’s Diabetes-Promoting Actions
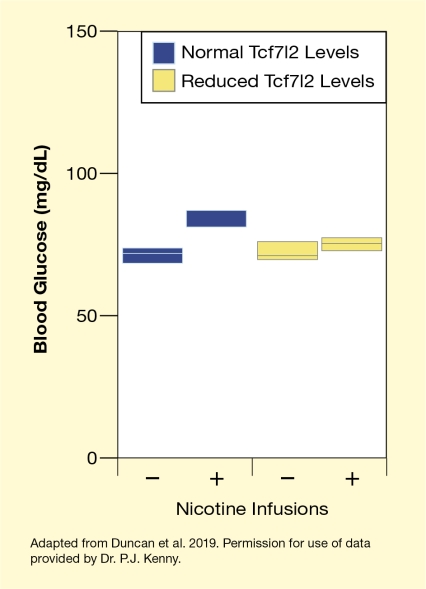
Another set of experiments using rats genetically modified to produce less Tcf7l2 protein explored how changes in Tcf7l2 expression altered nicotine’s effects on blood sugar regulation. Normally, nicotine can induce elevations in blood sugar levels (i.e., a hyperglycemic response). Additionally, repeated exposure to nicotine’s hyperglycemic actions leads to increased levels of the blood sugar-regulating hormones glucagon and insulin as well as diabetes-like dysregulation of blood sugar levels in the animals. The new study found, however, that rats with reduced Tcf7l2 activity in the medial habenula had a weaker hyperglycemic response to nicotine (see Figure 2). Moreover, the diabetes-promoting effect of repeated nicotine exposure was absent in Tcf7l2-deficient animals.
Dr. Kenny describes the implications of these findings as “quite far reaching” because the data suggest that smoking-related diseases associated with peripheral organ dysfunction, such as diabetes, may actually originate in the brain and not in the affected organ systems in people who smoke tobacco. Additionally, the findings raise the intriguing possibly that alterations in peripheral organ function can “feed back” to the brain to modulate nicotine’s addictive properties. Explains Dr. Kenny, “In effect, we have a yin-yang process whereby the brain controls peripheral diseases, and peripheral organs can regulate the addictive properties of nicotine. So, we have to view addiction and addiction-related diseases from a holistic, whole-organism perspective.”
This study was supported by NIDA grants DA020686 and DA035756.
- Text Description of Figure 1
-
The bar chart illustrates nicotine self-administration in rats with a disrupted Tcf7l2 gene and in control rats with an intact Tcf7l2 gene. Red bars represent the rats with a modified Tcf7l2 gene and blue bars represent the control animals. The horizontal x-axis shows the number of nicotine infusions per session that the rats self-administered on a scale from 0 to 30. The vertical y-axis shows the different nicotine doses in mg/kg that were administered with each infusion. Without nicotine in the infusion solution (top pair of bars), both groups of animals self-administered about five infusions per session. At a nicotine dose of 0.003 mg/kg per infusion (second pair of bars), control rats self-administered about 13 infusions and Tcf7l2-deficient rats administered about 20 infusions. At a nicotine dose of 0.01 mg/kg per infusion (third pair of bars), control rats administered about 15 infusions, and Tcf7l2-deficient rats administered about 27 infusions per session. At a nicotine dose of 0.03 mg/kg per infusion (fourth pair of bars), control rats administered about 12 infusions, and Tcf7l2-deficient rats administered about 14 infusions. At a nicotine dose of 0.09 mg/kg per infusion (fifth pair of bars), control rats administered about six infusions, and Tcf7l2-deficient rats administered about nine infusions per session. At a nicotine dose of 0.18 mg/kg per infusion (bottom pair of bars), control rats administer about 2.5 infusions, and Tcf7l2-deficient rats administered about six infusions per session.
- Text Description of Figure 2
-
This figure illustrates the changes in blood glucose levels (hyperglycemic response) following nicotine infusions in rats with reduced Tcf7l2 levels (yellow bars) and control rats with normal Tcf7l2 levels (blue bars). The horizontal x-axis indicates whether nicotine infusions were administered (+) or not (–). The vertical y-axis indicates blood glucose levels on a scale from 0 to 150. In the control rats, blood glucose levels in the absence of nicotine (left bar) ranged from about 70 mg/dL to about 75 mg/dL; after nicotine infusion (second bar from the left), blood glucose levels increased to about 80 mg/dL to about 85 mg/dL. In the Tcf7l2-deficient rats, blood glucose levels in the absence of nicotine (second bar from the right) ranged from about 71 mg/dL to about 77 mg/dL, and after nicotine infusion (right bar), they ranged from about 73 mg/dL to about 78 mg/dL.
Source:
- Duncan, A., Heyer, M.P., Ishikawa, M., et al. Habenular TCF7L2 links nicotine addiction to diabetes. Nature. 574:372-377, 2019.