This study reported that:

- Mice that were exposed to nicotine during early development and their offspring showed changes in the levels of epigenetic factors that regulate gene expression in certain brain regions.
- The observed changes were consistent with altered patterns of epigenetic modifications seen in mice and humans with neurological and behavioral disorders.
- Epigenetic changes that can persist for generations after nicotine exposure may predispose children and grandchildren of people who smoke to develop neurological and behavioral disorders.
Maternal smoking of traditional and electronic cigarettes during pregnancy exposes the fetus to nicotine; developmental nicotine exposure (DNE) can harm the baby’s brain development and has been linked to disorders such as attention deficit/hyperactivity disorder, autism, and schizophrenia in children. Of particular concern, animal model studies of maternal and grandmaternal cigarette smoking or vaping nicotine reveal that DNE is associated with behavioral and neurological changes that can persist across multiple generations. A recent NIDA-funded study by Dr. Jordan M. Buck and colleagues at the University of Colorado has identified a mechanism that may be responsible for this intergenerational transmission. The researchers focused on epigenetic changes—chemical modifications of the DNA and associated proteins that influence gene expression. “Our findings imply that epigenetic perturbations may constitute a nexus for intergenerational transmission of DNE-induced neurodevelopmental deficits,” says Dr. Buck.
Epigenetic Modifications Regulate Gene Expression
Epigenetic modifications involve the addition or removal of specific chemical groups (e.g., methyl or acetyl groups) to the DNA or the histone proteins around which the DNA is wrapped. These modifications are performed by specific epigenetic factors (see textbox) and can promote or block gene expression. For example, addition of methyl groups (i.e., methylation) of specific genes can prevent expression of these genes. These regulatory processes can be limited to specific tissues and cells and can be induced or altered by exposure to drugs. For example, children with DNE or animal models of DNE exhibit changes in DNA methylation, and thus altered gene expression, in the brain’s dorsolateral prefrontal cortex. These alterations are associated with impaired neuronal signaling and neurobehavioral/neurodevelopmental outcomes. DNE in mice and smoking or vaping during pregnancy in humans also may trigger changes in the levels of epigenetic factors and co-factors that mediate DNA methylation and demethylation in the frontal cortex, striatum, and hippocampus.
Epigenetic Factors
Numerous factors (i.e., enzymes) are involved in epigenetic processes and thus affect gene expression. Dr. Buck and colleagues examined four factors:
- DNA methyltransferase 3A (DNMT3A), which adds methyl groups to DNA to suppress gene expression
- Ten-eleven translocase methylcytosine dioxygenase 2 (TET2), which promotes DNA demethylation to permit gene expression
- Histone deacetylase 2 (HDAC2), which removes acetyl groups from histones, thereby allowing DNA methylation and gene suppression
- Methyl-CpG-binding protein-2 (MeCP2), which binds to methylated DNA and recruits HDAC2.
The activity of HDAC2 and MeCP2, in turn, can be regulated by the addition of phosphate groups.
Changes in Epigenetic Factors Can Be Transmitted to Later Generations
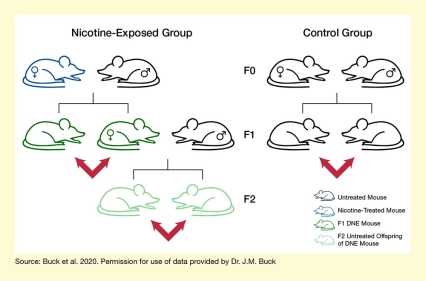
In previous work, Dr. Buck and colleagues detected abnormal DNA methylation patterns in second-generation offspring of mice exposed to nicotine. In their most recent study, the team further investigated whether changes in the levels and activity of specific epigenetic factors in the DNE mouse brain could also be transmitted across generations. They exposed female mice to either a nicotine-plus-sugar solution or a sugar-only solution in their drinking water for 30 days before mating them with nonexposed male mice. The treatment continued during the females’ pregnancy and until the offspring were weaned. First-generation (F1) female offspring from the nicotine-exposed group were mated with untreated males to produce second-generation (F2) offspring (see Figure 1). The researchers collected samples from the frontal cortex, striatum, and hippocampus from the F1 of the control or nicotine-exposed mice and from the F2 offspring of the nicotine-exposed mice. They then analyzed the levels of the DNMT3A, TET2, MeCP2, and HDAC2 epigenetic factors in these samples, as well as the phosphorylated forms of MeCP2 and HDAC2.
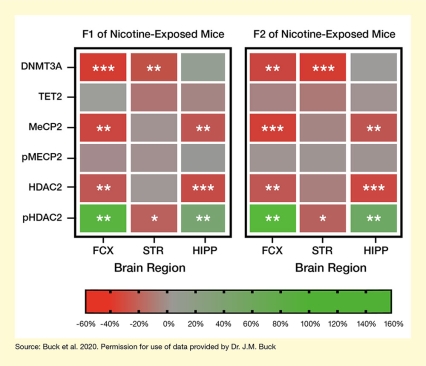
Compared with the F1 offspring of the control females, both F1 and F2 offspring of the nicotine-exposed females showed significant changes in the abundance of several epigenetic factors in specific brain regions (see Figure 2). Moreover, the observed changes were similar in both F1 and F2 offspring of nicotine-exposed mice. They included:
- Significantly decreased levels of DNMT3A in the frontal cortex and striatum.
- Decreased levels of MeCP2 and HDAC2 in the frontal cortex and hippocampus.
- Increases in phosphorylated HDAC2 in the frontal cortex and hippocampus.
Maternal nicotine exposure did not alter TET2 or phosphorylated pMeCP2 levels.
These changes in epigenetic factor levels match changes in DNA modifications observed previously in DNE mice. For example, the research team found DNMT3A deficits in the frontal cortex and striatum of DNE mice—regions where earlier studies had detected less DNA methylation. This suggests that DNE-induced reductions in DNMT3A levels that are passed on from one generation to the next may be responsible for reduced methylation in these brain areas.
Similar changes in epigenetic factors—and in the DNA modifications they cause—have also been found in mice and humans with various neurobiological and behavioral disorders. “Our findings contribute to a growing body of evidence for an epigenetic mechanism linking neurodevelopmental disorders to maternal and grandmaternal smoking,” says Dr. Buck. Although it remains unclear how nicotine exposure elicits changes in epigenetic factor levels and how these changes are passed on across generations, the new study’s findings may inform development of novel targeted therapeutics and the implementation of public health measures to mitigate the risks associated with maternal and grandmaternal smoking.
This work was supported by NIDA grants DA040228 and DA017637.
- Text Description of Figure 1
-
The figure shows the experimental design to analyze effects of developmental nicotine exposure over two generations. The top row of mice indicates the parent, or F0 generation; the middle row indicates the first-generation offspring, or F1 generation; and the bottom row indicates the second-generation offspring, or F2 generation. The left panel of the figure indicates the design for nicotine exposure. The blue mouse at the top left is the nicotine exposed female, black mice indicate non-nicotine-exposed males. The dark green mice in the middle row represent F1 offspring whose mother had been treated with nicotine, and light green mice in the bottom row indicate F2 offspring whose maternal grandmother was had been treated with nicotine. The right panel indicates control animals, none of which were exposed to nicotine. Red arrows point to the animals that were analyzed further.
- Text Description of Figure 2
-
The figure indicates the effect of nicotine exposure of female mice on epigenetic factors in F1 offspring (left panel) and F2 offspring (right panel). In each panel, the left column represents samples from the frontal cortex, the middle column represents samples from the striatum, and the right column represents samples from the hippocampus. The six rows in each panel represent the different epigenetic factors analyzed. A color bar at the bottom represents the levels of the epigenetic factors compared with those in F1 offspring of control animals on a scale from -60% to +160%. Reduced levels compared with control animals are indicted by red shading, similar levels to control animals are indicated by gray shading, and increased levels compared with control animals are indicated by green shading. One, two, or three asterisks within a field indicate differences at varying levels of statistical significance.
Expression of DNMT3A (row 1) was significantly reduced in the frontal cortex and striatum of both F1 and F2 offspring nicotine-exposed mice. Expression of MeCP2 (row 3) and HDAC2 (row 5) was significantly reduced in the frontal cortex and hippocampus of both F1 and F2 offspring of nicotine-exposed mice. Expression of pHDAC2 was significantly increased in the frontal cortex and hippocampus, and significantly decreased in the striatum, of both F1 and F2 offspring of nicotine-exposed mice. Expression of TET2 (row 2) and pMECP2 (row 4) did not differ significantly between F1 or F2 offspring of nicotine-exposed mice and F1 offspring of control mice in any of the brain regions analyzed.
Source
- Buck, J.M., O’Neill, H.C., Stitzel, J.A. Developmental nicotine exposure engenders intergenerational downregulation and aberrant posttranslational modification of cardinal epigenetic factors in the frontal cortices, striata, and hippocampi of adolescent mice. Epigenetics Chromatin. 2020;13(1):13. doi: 10.1186/s13072-020-00332-0.