Researchers have long focused on motivation as the centerpiece of the addiction puzzle, based on the observation that in many addicted individuals, compulsive drug-seeking behavior overtakes the most fundamental motivators, including food and sex. Now, however, researchers are beginning to examine another aspect of addictive drugs—their powerful and long-lasting effects on mood. People who have recently stopped abusing stimulant drugs commonly experience "abstinence syndrome": low energy, irritability, restlessness, an inability to feel pleasure, and problems with concentration. Anxiety and panic attacks also are sometimes associated with cocaine abstinence. Addiction researchers are examining the neurobiology underlying abstinence syndrome with an eye toward improving current therapies' ability to alleviate these symptoms and prevent relapse.
NIDA investigators concentrated recently on the impact of cocaine on the neurotransmitter norepinephrine (NE), one of the two neurochemicals most responsible for mood. Stimulation of brain cells by serotonin and NE is central to positive mood, feeling energetic, and maintaining focus as well as sleep, appetite, and coping with stress. Two recent animal studies, one conducted by investigators at NIDA's Intramural Research Program (IRP) in Baltimore and another by NIDA-funded researchers at Harvard Medical School's New England Primate Research Center in Southborough, Massachusetts, suggest that cocaine may compromise NE's ability to stimulate brain cells by altering a communication protein, called the α2-adrenergic receptor, on the surfaces of the cells.
In the Baltimore study, Dr. Michael Baumann and colleagues hypothesized that by giving rats cocaine regularly—twice-daily injections at 15 mg/kg of the animals' weight for 7 days—and then abruptly stopping it, they would reduce the α2-adrenergic receptors' responsiveness. To assess the adrenergic system in the cocaine-exposed, now "abstinent" rats, the researchers used the clonidine challenge procedure, which indirectly indicates α2-adrenergic receptor activity by measuring how much plasma levels of growth hormone (GH) increase following exposure to the drug clonidine (see "Clonidine Challenge Suggests That Cocaine Abuse Desensitizes Adrenergic System"). Confirming the researchers' hypothesis, the cocaine-exposed animals showed a blunted GH response—less than half that of saline-exposed animals—15 and 30 minutes after the clonidine challenge. The rats' response was still low, but returning toward normal, when the researchers repeated the challenge procedure 8 days after daily injections stopped.
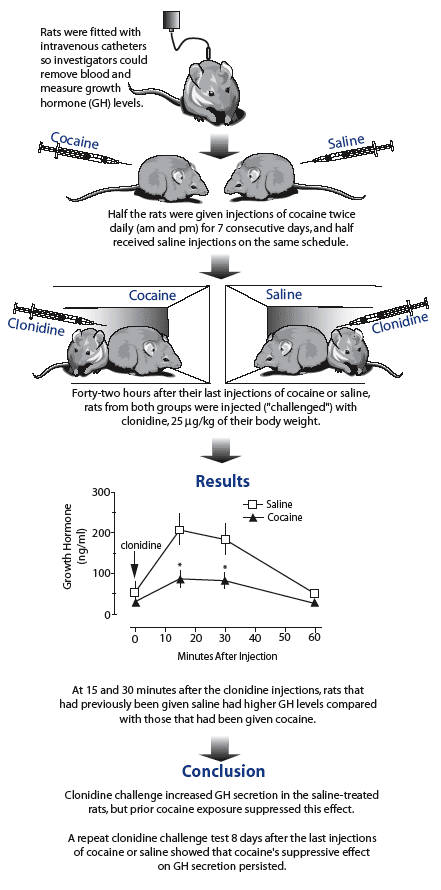 Clonidine Challenge Suggests That Cocaine Abuse Desensitizes Adrenergic System. Clonidine elevates growth hormone (GH) secretion in rats, a response mediated through the α2-adrenoreceptors in the brain. When rats are exposed to repeated cocaine injections, GH secretion in response to clonidine is lowered. The clonidine challenge test is used to measure α2-adrenoreceptor sensitivity in humans as well as animals.
Clonidine Challenge Suggests That Cocaine Abuse Desensitizes Adrenergic System. Clonidine elevates growth hormone (GH) secretion in rats, a response mediated through the α2-adrenoreceptors in the brain. When rats are exposed to repeated cocaine injections, GH secretion in response to clonidine is lowered. The clonidine challenge test is used to measure α2-adrenoreceptor sensitivity in humans as well as animals.The findings suggest that cocaine consumption and cessation may lower recovering individuals' moods by desensitizating the α2-adrenoreceptors, but the results are preliminary. "The adrenergic system is complex, with multiple pathways in the brain and ," says Dr. Baumann. "We still have much to learn about how drug exposure affects all these pathways, how it affects serotonin, and how they both influence growth hormone."
People with depression secrete less GH in response to the clonidine challenge than do those without the condition, a clinical finding that suggests possible links between NE receptor function, mood disorders, and cocaine withdrawal. "Although investigators are only beginning to characterize norepinephrine's role in addiction, a growing of animal and clinical research suggests important connections between the adrenergic system, mood and anxiety disorders, and the depression-like symptoms experienced by people trying to overcome cocaine addiction," Dr. Baumann says.
A Role in Relapse?
In a study that explored the chemical basis of mood and cocaine relapse, Dr. Roger Spealman and colleagues hypothesized that blocking α2-adrenergic receptors in monkeys would generate anxiety and induce a resumption of previously extinguished cocaine-seeking behavior.
The researchers trained monkeys to seek cocaine by pressing a lever. When the monkeys reached a high rate of lever pressing, the researchers disconnected it from the injection device. The monkeys kept trying the lever for a while, but with no more cocaine forthcoming, gradually left off. The investigators hypothesized that giving monkeys a drug to reduce NE activity would make the animals anxious, and the anxiety would intensify their urge for cocaine to the point where they would resume pressing the lever despite recent experience of its futility.
Dr. Spealman and colleagues gave the now "abstinent" animals various doses of two α2-adrenergic blocking agents, yohimbine and RS-79948, in separate test sessions. Both α2-adrenergic receptor blockers set the animals to pressing the lever again. The increase ranged from 1.5 to 4 times the response to injections of sterile water, depending on the dose and drug. The yohimbine injections also increased physiological and behavioral signs of anxiety: salivary cortisol levels and self-grooming and scratching.
To confirm that yohimbine's behavioral effects were due to its inhibition of the α2-adrenergic system, rather than any of the other neurotransmitter systems this agent affects, the researchers conducted further experiments. First, they gave the monkeys yohimbine plus clonidine, a drug that selectively blocks yohimbine's effects on the α2-adrenergic receptors. With this regimen, the animals resumed lever pressing hardly or not at all. Next, the researchers gave the monkeys yohimbine plus flupenthixol, a drug that reduces dopamine activity and has no effect on yohimbine's inhibition of α2-adrenergic activity. Under this regimen, the animals did resume lever pressing. Both of these findings pointed to α2-adrenergic suppression as the key to yohimbine's effects in the first experiment. Yohimbine did not stimulate movement or make the animals restless, which indicates that it worked by blocking receptors, not simply by mimicking the stimulant effects of cocaine.
"It makes sense physiologically that the adrenergic system might play a role in addiction; cocaine activates norepinephrine as much as it stimulates dopamine," says Dr. Spealman. Work by others suggests that cocaine abuse leads to long-term desensitization of the NE system in areas of the brain involved in reward and stressinduced reinstatement of drug-seeking. "This has led some researchers to speculate that the desensitized adrenergic system increases vulnerability to further norepinephrine disturbances—for example, those caused by stress or drug re-exposure—which may increase relapse risk," explains Dr. Spealman.
Researchers continue to seek to unravel the complexities underlying withdrawal and relapse to drug-taking. If, as scientists now think, these phenomena arise from sequential alterations in both the reward and mood pathways, "addiction medications may have to target different neurotransmitters at various stages of abstinence," says Dr. Minda Lynch of NIDA's Division of Basic Neuroscience and Behavioral Research.
Sources
- Baumann, M.H.; Milchanowski, A.B.; and Rothman, R.B. Evidence for alterations in α2-adrenergic receptor sensitivity in rats exposed to repeated cocaine administration. Neuroscience 125(3):683-690, 2004. [Abstract]
- Lee, B.; Tiefenbacher, S.; Platt, D.M.; and Spealman, R.D. Pharmacological blockade of α2-adrenoreceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology 29(4):686- 693, 2004. [Full Text]
