Dr. Linda Dwoskin
Dr. Linda DwoskinPharmacologist Dr. Linda Dwoskin and her research team have generated a dozen compounds that they believe might help people overcome addiction to methamphetamine. Over the coming years, Dr. Dwoskin plans to single out the most promising one, complete the requirements for obtaining approval from the Food and Drug Administration to test it in patients, and then evaluate it in clinical trials.
Depending on the outcome of those trials, that compound could become the first proven effective medication for treating methamphetamine addiction. Or, Dr. Dwoskin may find that she has simply reached yet another branching in a research and development path that she has followed, to date, for nearly 20 years.
This is the first in a series of NIDA Notes articles that will follow Dr. Dwoskin and her team as their project unfolds. We will accompany the researchers as they formulate hypotheses, plan and carry out experiments to test the hypotheses, and assess the results. Dr. Dwoskin and her colleagues will share their motivations, reasoning, tools, setbacks, and successes. Our aim is to bring readers into direct contact with drug abuse research as it happens, show the idealism and skill of researchers, and share the excitement and suspense of the quest to relieve suffering.
“Aha!”
In 1994, Dr. Dwoskin and colleagues at the University of Kentucky were seeking new medications to treat nicotine addiction. They decided to map the pharmacology of a compound called lobeline, which had appeared to help smokers quit in some studies but not others. Perhaps they could identify the property that accounted for lobeline’s partial success and then modify the molecule to accentuate it.
The mapping results didn’t advance lobeline’s prospects as a smoking cessation agent. However, they revealed that the compound had a previously unrecognized pharmacological action: It interacts with a structure called the vesicular monoamine transporter 2 (VMAT2).
Dr. Dwoskin had prior acquaintance with VMAT2. In her postdoctoral studies at the University of Colorado, she had learned that methamphetamine interacts with this same protein.
“That was a bit of serendipity,” says Dr. Dwoskin. “I realized that lobeline was like methamphetamine in this one way, but different in other ways.”
One difference stood out: When methamphetamine interacts with VMAT2, it causes the user to experience a sensation of reward—the high that motivates repeated use of the stimulant (see “VMAT2, Key to the Methamphetamine High”). In contrast, animal studies had established that lobeline does not confer reward.
At that point, says Dr. Dwoskin, “I had a real ‘Aha’ moment.” She saw that lobeline might make an effective medication to treat methamphetamine dependence. The strategy would be to give patients lobeline to occupy VMAT2 and thereby obstruct methamphetamine from interacting with the protein. Without access to its target, the stimulant would not produce its high.
The strategy of using a non-rewarding molecule to deny an addictive drug access to its site of action is well established. For example, naltrexone prevents alcohol and opioid drugs from linking to opioid receptors in the brain. By doing so, it reduces abstinent individuals’ risk for relapse to use of those addictive substances.
Would lobeline similarly benefit people with methamphetamine addiction?
“Ugh!”
Dr. Dwoskin and her colleagues set about evaluating their concept in a series of laboratory studies. One result was key: In rats, lobeline indeed suppressed methamphetamine’s rewarding effect. Animals initially pressed a lever to obtain methamphetamine, but stopped doing so after being injected with lobeline. Moreover, this effect didn’t wear off, but persisted with repeated injections. This latter finding was important, because many patients who use a medication to support abstinence will need to continue taking it for extended periods.
In 2003, Dr. Dwoskin had sufficient evidence supporting lobeline to approach NIDA with a proposal to test it in a clinical trial. The trial would aim to determine whether lobeline enhanced patients’ outcomes in treatment for methamphetamine addiction.
Dr. Dwoskin proposed that there was no need to perform a preliminary trial to test whether people might safely take lobeline. She pointed out that Native Americans have been making medicinal use of the Lobelia plant, which is the source of lobeline, for centuries. Moreover, other researchers had conducted successful clinical safety trials of lobeline while investigating it as a potential aid to smoking cessation.
NIDA wanted assurance, however, that lobeline would be safe specifically for methamphetamine users. They were concerned that the compound might interact with methamphetamine to produce adverse effects. Moreover, methamphetamine users have higher than average rates of serious health problems, and so might be particularly vulnerable to toxic effects from any medication. For example, methamphetamine is cardiotoxic, and both human immunodeficiency virus (HIV) disease and hepatitis are highly prevalent among users of the drug.
Dr. Dwoskin and NIDA enlisted clinician-researcher Dr. Reese Jones of the University of California in San Francisco (UCSF) to manage the safety trial. Ten methamphetamine-addicted volunteers took lobeline under close monitoring in an inpatient setting for several days.
The trial affirmed that methamphetamine users can safely take lobeline. As the researchers hoped, no volunteer experienced any serious adverse effect from the compound.
But there was a problem: The volunteers hated the taste of lobeline. “Lobeline is a very bitter tasting plant alkaloid,” says Dr. Dwoskin. “The lobeline we used in our trial was in the form of a sublingual tablet that patients dissolved under the tongue. I tasted lobeline myself: It was disgusting. To make matters worse, lobeline has a short duration of action, so patients needed to take it several times a day.”
So strong was the volunteers’ aversion to lobeline’s taste that Dr. Dwoskin and her team felt that sublingual lobeline would be untenable for ordinary clinical use: “Our consensus was that our volunteers had stuck it out because they were in the trial. But if patients outside of the research setting were given a prescription to take it on their own, many would not do so.”
Dr. Dwoskin and colleagues considered and rejected the idea of mixing lobeline with another compound to mask its taste. Even so, many patients would surely have difficulty adhering to a regimen of multiple doses per day. Reformulating lobeline as an extended-release injectable would circumvent both problems, but injectables have convenience and usability issues of their own.
For the first time but not the last, Dr. Dwoskin decided that a compound in which they had seen great promise, and which they had developed with high hopes, was not suitable for clinical use.
Setback and Reset
Dr. Dwoskin returned to the drawing board, but not to square one. The work that she and her colleagues had done with lobeline had confirmed that its interaction with VMAT2 suppressed methamphetamine’s rewarding effect. They also had shown that concurrent exposure to a compound that interacts with VMAT2 and methamphetamine does not produce dangerous health effects.
The research team now set its sights on creating a novel compound that would interact similarly to lobeline at VMAT2, and would preserve or improve upon lobeline’s desirable properties while shedding its negative features. Although lobeline had fallen short, the information that the researchers had amassed about its structure and pharmacological activity would be invaluable. They would start with the lobeline molecule itself and modify it in ways that this knowledge suggested might take them to their goal.
“The lobeline clinical trial in a way was a setback,” says Dr. Dwoskin. “But I was not discouraged. I thought we could come up with a better compound.”
The research pathway Dr. Dwoskin and her colleagues now embarked on was much longer than the one she envisioned when she thought lobeline might succeed to clinical use. Ten years later, she is still not to the end of it. Along the way she and her colleagues have created more than a thousand compounds that interact with VMAT2, and experienced high points and near misses—which we’ll discuss in the next article in this series.
Throughout, Dr. Dwoskin has never lost her enthusiasm. “I think that as a person who is trying to do drug discovery, you go into it knowing that it is going to be a long haul, and that you have to be persistent and devoted to accomplishing the goal,” she says. “And the goal in this case is particularly powerful: To help people who are suffering because they can’t stop taking methamphetamine, who get into all kinds of trouble, lose their families and jobs, and have nothing to turn to. Because, in terms of a medication, to date there really is nothing for them.”
Read Part 2, Part 3, and Part 4 of this Narrative of Discovery.
VMAT2, Key to the Methamphetamine High
Drugs of abuse make users “high” by flooding the brain’s reward center with the neurochemical dopamine. Methamphetamine and other amphetamine drugs cause dopamine flooding by interacting with a protein called the vesicular monoamine transporter 2 (VMAT2).
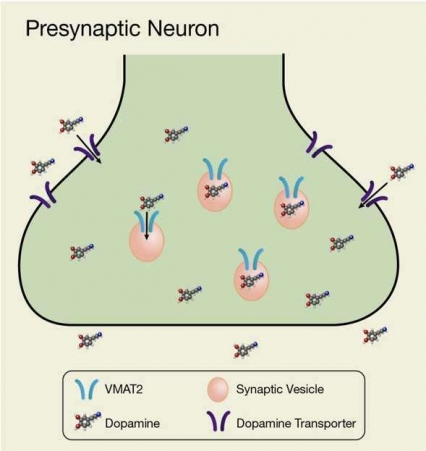
- Text Description of Graphic
-
The graphic is a schematic, showing a presynaptic terminal of a dopaminergic neuron. Dopamine molecules located outside of the presynaptic terminal are taken up into the neuron through the dopamine transporter in the neuron’s membrane. Dopamine that has entered the presynaptic neuron is then taken up through the VMAT2 protein into small synaptic vesicles inside the neuron. VMAT2 thereby acts as a channel that facilitates the transfer of dopamine from the cytosol into the vesicles.
VMAT2 resides in the membrane that surrounds vesicles, which are pouch-like structures that circulate in the fluid (cytosol) that fills the interior of neurons (see Figure). VMAT2 draws free-floating dopamine molecules from the cytosol into the vesicles for storage. In a drug-free brain, this activity of VMAT2 helps maintain a healthy balance between free dopamine in the cytosol and stored dopamine inside the vesicles.
Methamphetamine’s interaction with VMAT2 inhibits the transporter from drawing dopamine into the vesicles and causes the vesicles to release stored dopamine. Neurotransmitter accordingly builds up in the cytosol, giving rise to two undesirable consequences:
- The neuron offloads large quantities of excess cytosolic dopamine into the brain’s reward center, precipitating the drug high
- Cytosolic dopamine nevertheless remains elevated and can reach levels that are toxic to the neuron