 Dr. Linda Dwoskin and Dr. Peter A. Crooks
Dr. Linda Dwoskin and Dr. Peter A. CrooksIn Part 1 of this series:
While investigating the potential of a compound called lobeline to treat nicotine addiction, pharmacologist Dr. Linda Dwoskin had an insight. She saw that lobeline is like methamphetamine in one way, and unlike it in another: Like, in that both interact with an intracellular protein called the ventricular monoamine transporter 2 (VMAT2); unlike, in that lobeline does not cause euphoria or addiction.
Dr. Dwoskin hypothesized that lobeline might block methamphetamine’s access to VMAT2 and, by doing so, prevent the drug’s addictive effects. Over the next decade, she and her team put lobeline through extensive laboratory and animal tests. The results were encouraging: Lobeline reduced animals’ responses to methamphetamine and raised no red flags for safety.
The next step was to assess lobeline’s safety for methamphetamine users. The compound passed this test, too, producing no significant adverse effects in a small group of volunteers. However, the study also terminated Dr. Dwoskin’s hopes for lobeline. Volunteers found its taste revolting and resisted taking it, especially as its sublingual formulation required them to dissolve it slowly under the tongue. Dr. Dwoskin and her team concluded that there was little point in developing a medication that few patients would take willingly.
This article takes up Dr. Dwoskin and her project as she enters a new research phase. So far, she has tried to advance an existing compound through the stages of testing required to prove its worth as a medication. Now, she takes on the more elaborate challenge of constructing a new compound that reproduces lobeline’s potentially therapeutic activity at VMAT2, without its undesirable effects.
First Strike, Lucky Strike
“Lobeline’s failure was a blow,” says Dr. Dwoskin. “But I was still very invigorated by the idea that we had a novel medication target in VMAT2. Besides, I thought all along that we could design a much better medication than lobeline.”
While Dr. Dwoskin and her team still hoped that lobeline would have clinical use, they were already thinking about ways to improve upon it. They envisioned a compound that would:
- Interact more strongly with VMAT2—to produce beneficial effects at lower dosage levels.
- Interact with VMAT2 alone, avoiding lobeline’s concurrent action at nicotinic and other receptors—to limit nontherapeutic, and potentially adverse, effects.
- Have greater chemical stability—to allow manufacture in pill form.
- Have a longer half-life—to reduce the frequency of dosing needed to maintain therapeutic levels in the patient’s brain.
To co-lead the effort to create this ideal compound, Dr. Dwoskin turned to Dr. Peter A. Crooks. A medicinal chemist, Dr. Crooks’ stock in trade is identifying, synthesizing, and modifying chemical compounds for pharmaceutical use.
To begin, the researchers consulted a library of information on the compounds, about a dozen in number, that are produced, like lobeline, by the Lobelia plant species. Dr. Crooks says, “Ever since these plants were first discovered a couple of centuries ago, people have been investigating the compounds they produce.” By comparing and contrasting these related compounds, Drs. Dwoskin and Crooks formed initial suppositions as to which structural features contribute to their various pharmacological properties.
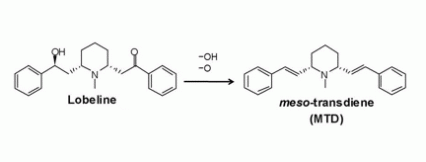 Figure. From Lobeline to Meso-Transdiene Removing the hydroxyl (OH) group and the oxygen (O) atom from the lobeline molecule yielded meso-transdiene (MTD). Dr. Crooks anticipated that stripping away the two oxygen functionalities would produce a more stable molecule than lobeline, and it did. Dr. Crooks was happily surprised that the modification also eliminated a problematic property of lobeline, activity at the nicotinic receptor.
Figure. From Lobeline to Meso-Transdiene Removing the hydroxyl (OH) group and the oxygen (O) atom from the lobeline molecule yielded meso-transdiene (MTD). Dr. Crooks anticipated that stripping away the two oxygen functionalities would produce a more stable molecule than lobeline, and it did. Dr. Crooks was happily surprised that the modification also eliminated a problematic property of lobeline, activity at the nicotinic receptor.
- Text Description of Figure
-
The graphic compares the molecular structures of lobeline and its reduced version, meso-transdiene (MTD). The two molecules have similar general structures, sharing 21 carbon molecules in two carbon chains and two phenyl rings, represented by hexagons, that flank a central hexagon ring containing a nitrogen molecule. However, MTD lacks lobeline’s two oxygen groupings or “functionalities,” one an OH, or hydroxyl group, and the other a double-bonded oxygen group. Each of these groupings are replaced by a double-bond in the carbon chain on either side of the central ring. Removal of the oxygen functionalities made the MTD molecule more stable and eliminated its activity at nicotinic receptors, a possible source of the medication’s side effects.
Based on this research and his own long experience tweaking molecules, Dr. Crooks suggested that that they remove lobeline’s two oxygen “functionalities”, or groupings (See Figure).
The maneuver succeeded beyond the researchers’ imagining. Dr. Crooks hypothesized that the reduced molecule, called meso-transdiene (MTD), would be more stable than lobeline. It was. Unexpectedly, and happily, it also lacked lobeline’s affinity for nicotinic receptors.
“Eliminating lobeline’s activity at nicotinic receptors was a terrific bonus,” Dr. Dwoskin says. “First, we could now definitely show that lobeline’s inhibition of VMAT2 is the activity that counters methamphetamine’s effects. Before, we could never be sure that lobeline’s interaction with nicotinic receptors wasn’t responsible. Second, lobeline’s activity at nicotinic receptors had been a potential source of side effects. For example, we suspected that it contributed to nausea that some patients experienced in our clinical trial.”
Reflecting on that moment, Dr. Dwoskin says, “Serendipity got me started on this line of research, and it has been our friend, thus far, all along the way. When you mess with a molecule you don’t know what’s going to happen. But one of the very first manipulations we tried unexpectedly accomplished not one, but two of our goals for improving lobeline.”
Unfortunately, MTD’s promise proved to be short-lived. Further testing disclosed properties that made the compound unsuitable as a medication.
Nevertheless, the researchers were energized by the success of their first lobeline manipulation. Dr. Dwoskin says, “MTD was a turning point. We weren’t super disappointed that MTD didn’t work out, because we had gotten rid of the nicotinic receptor activity. We were spurred on with the hope that different alterations might lead to our ideal molecule.”
From Intuition to Induction
Drs. Dwoskin and Crooks ramped up their discovery process. To date, they have created and tested more than 1,500 lobeline analogs. Always preserving the central carbon and phenyl rings that constitute lobeline’s core, the chemists tinker with the rest—adding, subtracting, moving around, and building out atoms and groups of atoms.
At first, the researchers had little to guide them in deciding which of the vast number of possible alterations to the lobeline molecule might bring them closer to their goal. Despite the prior work on lobeline, information was scant about which of its parts influence the functional properties that they wished to improve. In addition, Dr. Crooks explains, “We had no information on the structure of our target molecule, VMAT2, so in that sense we were trying to fashion a key without knowing the shape of the lock. We had to solve it the hard way, which was to just make lots and lots of compounds and see how they performed.”
In line with that strategy, Dr. Crooks directed his group to produce only molecules that could be synthesized relatively easily. He explains, “With some more difficult molecules, it can take from 3 months to a year to execute the sequence of chemical reactions needed to synthesize a single compound. We try to avoid such compounds. We want to be nimble and create many molecules in a short time, so that we can build our structure-activity knowledge base and close in on our target.”
As Drs. Dwoskin and Crooks make observations on an ever-growing array of molecules, they enter the information into an artificial neural network (ANN). This computer algorithm has enabled them to move to a more guided and efficient discovery process.
ANNs mimic the human brain’s use of past experience to predict future events. In building their ANN, the researchers:
- Gave their computer an accounting of “past experience” by inputting all of their own ongoing and others’ previously recorded observations of lobeline analogs’ structures and activities.
- Programmed the computer to extract a small set of relevant structure-activity relationships from the observations, parallel to the way the brain deduces useful relationships from the entirety of its experience.
- Reprogrammed the computer with an algorithm comprised of the extracted relationships, parallel to the way the brain stores its deduced relationships for use in future situations.
With their ANN thus primed, the researchers input particulars of the structure of each new lobeline analog that they consider making. The ANN applies those extracted relationships and outputs an estimate of the odds that the compound will have enough desirable characteristics to be worth the effort and expense.
The Work Flow
Dr. Crooks supervises the synthesis of new lobeline analogs by graduate students and postdoctoral fellows at the College of Pharmacy at the University of Arkansas for Medical Sciences in Little Rock. He forwards each newly synthesized compound to Dr. Dwoskin in Kentucky, where graduate students and postdoctoral fellows in her laboratory conduct tests (See “A Roster of Preclinical Tests”).
The whole team convenes biweekly by video conference. “Everyone brings something special to the table that makes our team highly synergistic,” Dr. Dwoskin says. “We have chemists and pharmacologists and behaviorists, all coming together to try to solve problems.”
At the meetings, each team member reports their progress on their particular task, and timelines are adjusted. When a new molecule is ready, the team determines the roster of tests that will be performed on it and the timeline for evaluation.
When a molecule has been tested, the team decides what the next steps should be. If the molecule still shows promise or has properties that bear further exploration, they work out a schedule for further testing. Otherwise, they abandon it and discuss how to apply what they have learned to design new molecules with more desirable features.
Dr. Dwoskin relishes the days when she receives delivery of a newly synthesized compound. “When we’ve thought about a structural change, and decided to make a compound, and then it arrives, the actual solid compound, and we can test it—that’s pretty neat. And then, once we’ve done the testing, getting a story together about what we learned, and putting it into the scientific literature—that also feels good.” To date, the team has published more than 45 journal papers related to the project.
At any given time, the team has several molecules in various stages of testing. “It’s sort of like a race,” Dr. Dwoskin says. “One molecule will look good for a while and become the lead molecule. Then it will fail, and another will take the lead. You never can predict what is going to cause a molecule to fall by the wayside.”
The cycle of planning, synthesizing, and testing analogs produces moments of exhilaration and disappointment, often linked. Thus, Dr. Dwoskin was excited when she had her original insight regarding lobeline’s activity at VMAT2, and let down when lobeline’s awful taste and short half-life disqualified it for development as a medication.
Similarly, the team was elated when MTD blocked methamphetamine’s effects without activating nicotinic receptors. “That was really cool,” Dr. Dwoskin says. “It proved we were right about the role of VMAT2.” However, hopes for MTD were dashed because the compound had poor solubility, which meant that they could not fully evaluate its behavioral effects.
Although their breakthroughs so far have not produced a medication for methamphetamine addiction, the researchers believe that each one brings them closer to their goal. Fifteen years into her project, Dr. Dwoskin says, “The work takes hold of you. It’s like figuring out a puzzle—you have to keep going. You’re always thinking about your final objective, to help people recover from the terrible burden of methamphetamine addiction.”
A New Leap Forward
Coming into a team meeting in 2009, Dr. Dwoskin was focused on completing the testing of a compound called UKMH-106. A molecular cousin of MTD, UKMH-106 looked like it might be a winner. Like MTD, it strongly inhibited VMAT2, inhibited methamphetamine-induced dopamine release, and left most nicotinic receptors alone. UKMH-106 also avoided the two properties that ultimately disqualified MTD: It did not inhibit the dopamine transporter on the cell surface of dopamine neurons (which creates a risk for reinforcing and addictive effects), and it was readily soluble in water (which is a criterion for adequate dosing).
At the meeting, however, Dr. Nichole Neugebauer, a graduate student on the team, presented new data on another compound. The new compound was called lobelane, and the data were so exciting that the team decided to drop UKMH-106 and concentrate their main energies on developing it. Lobelane’s fate will be covered in the next article in this series.
Read Part 3 and Part 4 of this Narrative of Discovery.
A Roster of Preclinical Tests
For efficiency and economy, Dr. Dwoskin says, “We try to bring the most promising molecules to the top as fast as we can, and fail quickly on the ones that are not going to work out.” In line with that policy, she says, “We first test each compound to make sure that it interacts with VMAT2, because that’s where our proposed therapeutic effect will happen.” Additional preclinical tests assess early indicators of potential efficacy and suitability as a pharmaceutical product, and they look for red flags for potential toxicity. A compound that performs well on these tests will still require further testing before the Food and Drug Administration will approve it for testing in people.
| Test medium | Test | Desired outcome |
|---|---|---|
| In Vitro | VMAT2 functional assay | The compound blocks VMAT2, which is the activity that the researchers hypothesize will help people overcome methamphetamine addiction. |
| Functional assays at other neurotransmitter transporters, including those for dopamine, serotonin, norepinephrine, and nicotinic acetylcholine | The compound has little or no effect on these transporters, minimizing or ruling out these possible causes of side effects. Activity at the dopamine transporter is especially undesirable, because it can cause rewarding feelings that promote abuse. | |
| Screens for absorption, distribution, metabolism, excretion, and toxicity | The compound has favorable pharmacokinetic properties and the screens disclose no risk of toxicity. | |
| Cardiotoxicity related to the human ether-à-go-go related gene | A negative finding, indicating that the compound is unlikely to cause torsade des pointes, a potentially fatal cardiac arrhythmia. | |
| Animal Tissue | Effect on methamphetamine-evoked dopamine release | The compound inhibits methamphetamine-evoked dopamine release, suggesting that the compound can decrease methamphetamine reinforcement. |
| Whole Animal | Effect of peripherally administered compound on methamphetamine-induced increase in locomotor activation | The compound inhibits methamphetamine-induced locomotor activation without causing intolerance or toxicity. |
| Effect on methamphetamine-induced dopamine toxicity | Compound does not exacerbate methamphetamine damage to dopamine neurons. | |
| Effect of orally administered compound on methamphetamine-induced locomotor activation, and on intravenous methamphetamine self-administration | The orally administered compound inhibits methamphetamine-induced locomotor activation and reduces methamphetamine self-administration without causing toxicity or tolerance. | |
| Effect on cue-induced reinstatement of methamphetamine seeking | Animals treated with the compound respond less avidly than control animals to methamphetamine-associated environmental cues. |
