Can marijuana use put offspring at heightened risk for opiate addiction, even if the use stops before the offspring are conceived? Recent animal research by NIDA-supported scientists suggests that the answer may be yes.
Dr. Yasmin L. Hurd and colleagues at the Icahn School of Medicine at Mount Sinai in New York City showed that rats whose parents had been exposed as adolescents to the main psychoactive ingredient in marijuana sought heroin more vigorously than the offspring of unexposed animals. Although more research is needed to confirm and explain the findings, they are consistent with other studies suggesting that a parent’s history of drug use, even preconception, may affect a child’s brain function and behavior.
Lasting Imprint
Scientists have known for a while that drugs of abuse produce some of their effects epigenetically—that is, by increasing or decreasing the rates at which the body’s genetic machinery produces certain proteins. Researchers recently reported that some epigenetic changes produced by cocaine appear to be inherited and affect the behavior of subsequent generations. In that experiment, rats whose parents had been exposed to cocaine responded differently when introduced to the drug than did rats whose parents had not been exposed.
Dr. Hurd and colleagues hypothesized that rats whose parents were exposed as adolescents to the main psychoactive ingredient in marijuana (delta-9 tetrahydrocannabinol, or THC) would inherit epigenetic changes that would alter their responses to heroin. To test the hypothesis, the researchers injected adolescent male and female rats with THC for 3 weeks on an intermittent schedule (1.5 milligram per kilogram of body weight every 3 days) that corresponds to the amounts consumed by a typical recreational marijuana user. They waited 2 to 4 weeks for the drug to wash out of the rats’ bodies, then paired and mated them.
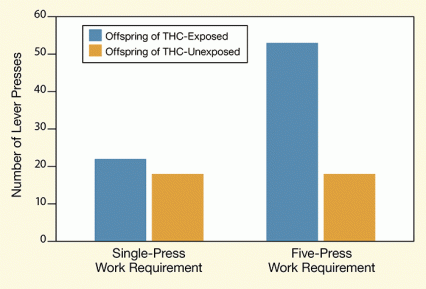 Figure 1. Offspring of THC-Exposed Parents Work Harder To Get Heroin When only a single press of a lever was required to obtain a dose of heroin, the offspring of THC-exposed and unexposed rats self-administered similar amounts of the drug. However, when the researchers raised the work requirement to 5 lever presses for a single dose, the rats whose parents had been exposed to THC pressed almost 3 times as often as the offspring of unexposed rats.
Figure 1. Offspring of THC-Exposed Parents Work Harder To Get Heroin When only a single press of a lever was required to obtain a dose of heroin, the offspring of THC-exposed and unexposed rats self-administered similar amounts of the drug. However, when the researchers raised the work requirement to 5 lever presses for a single dose, the rats whose parents had been exposed to THC pressed almost 3 times as often as the offspring of unexposed rats.
- Text Description of Figure 1
-
The figure shows a bar graph indicating the number of times rats pressed a lever to receive a heroin injection. In one set of experiments (indicated by bars on the left), the animals received a heroin injection after a single lever press, and in another (indicated by bars on the right), they had to press the lever 5 times to receive one heroin injection. The vertical (y)-axis shows the number of lever presses and the horizontal (x)-axis two groups of rats assessed for pressing of a lever that delivered heroin. One group, represented by light blue bars, consisted of animals whose parents had been exposed to THC and the other group, represented by orange bars, of a control group of rats whose parents had not been exposed to THC. When only a single lever press was required to receive heroin, both groups of rats pressed the lever with similar frequency (about 20 times in an experiment). When 5 lever presses were required for a heroin injection, the rats whose parents had been exposed to THC pressed the heroin lever on average almost 3 times as often (that is, more than 50 times) as the rats in the control group (less than 20 times).
When the offspring of these matings reached adulthood, the researchers presented them with a lever that, when pressed, delivered heroin (30 micrograms per kilogram of body weight). At first, the animals self-administered the drug at roughly the same rates as a group of control animals whose parents had not been exposed to THC. However, when the researchers made the animals work harder for the drug—requiring them to press the active lever at least 5 times to receive a dose—those whose parents had been exposed to the drug pressed on average nearly 3 times as often as the control rats (see Figure 1).
When the researchers removed the animals’ access to heroin, the THC-exposed rats’ offspring exhibited more pronounced withdrawal symptoms, such as increased locomotion and repetitive behaviors. Also during withdrawal, the two groups of rats differed in their readiness to approach a novel stimulus in their environment.
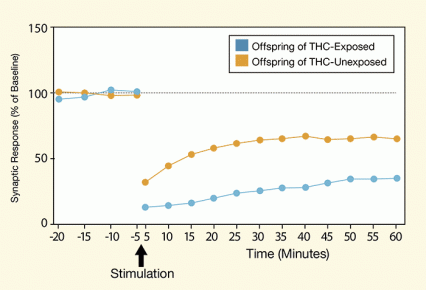 Figure 2. Offspring of THC-Exposed Rats Show Long-Term Depression of Synaptic Activity in the Striatum Medium spiny neurons in the dorsal striatum of rats whose parents had been exposed to THC responded less to electrophysiological stimulation than the neurons in rats whose parents had not been exposed to THC.
Figure 2. Offspring of THC-Exposed Rats Show Long-Term Depression of Synaptic Activity in the Striatum Medium spiny neurons in the dorsal striatum of rats whose parents had been exposed to THC responded less to electrophysiological stimulation than the neurons in rats whose parents had not been exposed to THC.
- Text Description of Figure 2
-
The figure shows a line graph of an experiment measuring long-term synaptic depression in medium spiny neurons. The vertical (y)-axis shows the synaptic response assessed as the percentage of activity relative to the activity in the neuron at baseline (before electrical stimulation of the neuron) and the horizontal (x)-axis the time in minutes on a scale ranging from 20 minutes before to 60 minutes after the stimulation. Two lines indicate synaptic responses in two groups of rats: one in which the rats’ parents had been exposed to THC (blue line and symbols) and another in which the rats’ parents had not been exposed to THC (orange line and symbols). Both animal groups showed very similar synaptic responses up until 5 minutes before the electrical stimulation, that is, around 100 percent, but differed in their responses after the stimulation. Five minutes after the stimulation, the synaptic activity was at its lowest in both groups and lower in the offspring of THC-exposed parents than in the control rats; synaptic activity was at approximately 10 percent in the offspring of THC-exposed parents and at approximately 30 percent in the control rats. Over a period of regular 5-minute measurements lasting until 60 minutes after the stimulation, synaptic recovery gradually recovered to an activity of approximately 30 percent in the offspring of the THC-exposed rats and to approximately 60 percent in the control rats.
Using electrophysiology, the researchers also demonstrated that the offspring of the THC-exposed rats had altered neuronal functioning (see Figure 2). The specific alteration that they observed—enhanced long-term synaptic depression of medium spiny neurons in the dorsal striatum—has been associated with addiction in previous studies. The neurons are less responsive to stimulation, which inhibits an individual’s ability to adjust to experience and results in habitual and compulsive behaviors rather than adaptive ones.
To identify the epigenetic factors that might underlie the differences they had observed in the offspring of the THC-exposed animals, the researchers assayed concentrations of messenger RNA (mRNA) for key proteins in the brain. The formation of mRNA is the first step in the process of protein production, and mRNA levels indicate how much protein is being produced at a given time. The researchers’ analysis showed that, during adolescence, the THC-exposed animals’ offspring had higher levels of mRNA for glutamate receptors and for the cannabinoid 1 receptor in the ventral striatum. During adulthood, the offspring of the THC-exposed rats had less mRNA for N-methyl-D-aspartate (NMDA)-type glutamate receptors in the dorsal striatum (see Figure 3). Reduced production of glutamate receptors could underlie the reduced responsiveness to stimulation researchers observed in that brain region.
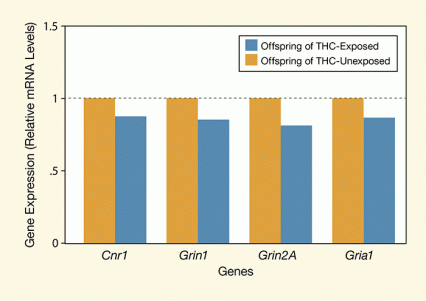 Figure 3. Offspring of THC-Exposed Parents Show Decreased Expression of Genes for Key Receptor Genes in the Brain Expression of genes for the glutamate-responsive receptors NMDA (Grin1 and Grin2A) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) (Gria1) and for the endocannabinoid receptor CB1 (CNR1) was lower in the dorsal striatum of adult rats whose parents had been exposed to THC. These changes in gene expression suggest an epigenetic effect of THC on glutamate and endocannabinoid signaling in the brain.
Figure 3. Offspring of THC-Exposed Parents Show Decreased Expression of Genes for Key Receptor Genes in the Brain Expression of genes for the glutamate-responsive receptors NMDA (Grin1 and Grin2A) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) (Gria1) and for the endocannabinoid receptor CB1 (CNR1) was lower in the dorsal striatum of adult rats whose parents had been exposed to THC. These changes in gene expression suggest an epigenetic effect of THC on glutamate and endocannabinoid signaling in the brain.
- Text Description of Figure 3
-
The figure shows a bar graph depicting the results measuring the expression of four genes in the dorsal striatum of rats whose parents had been exposed to THC and in a group of control rats whose parents had not been exposed to THC. The vertical (y)-axis shows the gene expression as mRNA levels calculated relative to mRNA levels in the control and the horizontal (x)-axis the four genes assessed, that is, CNR1, Grin1, Grin2A, and Gria1. As shown by blue bars representing expression in the offspring of THC-exposed rats, expression of all genes was decreased to approximately 80 to 90 percent of the expression in the control rats from parents that were not exposed to THC.
Is It Real?
The Mount Sinai researchers took pains to rule out potential nonepigenetic explanations for the differences they observed between their groups of rats. One concern was that the THC-exposed rats’ pups might themselves be exposed to the drug during gestation, resulting in altered brain development. To preclude this possibility, the researchers postponed mating their THC-exposed animals until sensitive gas chromatography and mass spectrometry confirmed that no drug remained in the animals’ blood or brain tissue. Another concern was that the THC-exposed animals might parent differently than the unexposed animals, potentially altering their offspring’s responses to heroin. To prevent this, the researchers removed the THC-exposed animals’ pups from their parents immediately after birth and had unexposed dams raise both groups of offspring in mixed litters.
Despite these careful controls, Dr. Hurd and colleagues say that they cannot completely rule out nonepigenetic explanations for the alterations they observed in their THC-exposed rats’ offspring until they see what happens in the next two generations of their germ line. The researchers are proceeding with this work.
“The idea of cross-generational transmission of complex traits such as drug responses without alterations to the genome is contentious,” says Dr. John Satterlee, Project Officer at NIDA’s Genetics and Molecular Neurobiology Research Branch. “Is it real? And if it’s real, how is it transmitted?” he asks.
Dr. Satterlee agrees with Dr. Hurd that studies on future generations are needed to definitively rule out the possibility that nonepigenetic factors led to the observed effects in the offspring. Previous exposure to THC theoretically could affect the womb or placental formation, he says, or lead to changes in the parents’ microbiome—the assemblage of microorganisms in the gut controlling a variety of conditions and behaviors—that were then transmitted to their offspring.
“If the effect is real, it’s important,” Dr. Satterlee says. “If studies show that marijuana use also shows cross-generational effects in people, those results would add to the known dangers of the drug and amplify the importance of prevention efforts, especially those aimed at youth,” he adds.
This study was supported by NIH grants DA030359 and DA033660.
Source
Szutorisz, H.; DiNieri, J.A.; Sweet, E. et al. Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology. 39(6):1315-1323, 2014. Abstract
