In this pilot study:
- Patients who received transcranial magnetic stimulation (TMS) were more likely to abstain from cocaine than patients who received medications for symptoms associated with abstinence.
- Researchers concluded that TMS appears to be safe and its efficacy as a treatment for cocaine addiction deserves to be evaluated in a larger clinical trial.
Transcranial magnetic stimulation (TMS) projects electromagnetic fields into the brain and can be used to either increase or decrease neuronal responsiveness in targeted brain areas. Researchers have hypothesized that administering TMS to strengthen activity in the prefrontal cortex (PFC) and downstream brain regions can alleviate cocaine addiction (see Narrative of Discovery: Can Magnets Treat Cocaine Addiction?). Previous findings that support the hypothesis include:
- Studies in animals and people have demonstrated that exposure to cocaine weakens neuronal activity in the PFC, and have linked that decreased activity to some of the primary manifestations of addiction, such as craving and compulsive drug-seeking.
- In a recent study, rats stopped seeking cocaine after researchers experimentally increased activity levels in their prelimbic cortex, a sub region of the rat cortex that shares functional similarities with the human dorsolateral PFC (see Prefrontal Cortex Stimulation Stops Compulsive Drug Seeking in Rats).
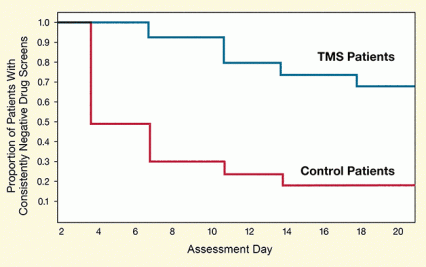 Figure. Patients Receiving TMS for Cocaine Addiction Achieve Higher Rates of Abstinence During a 21-day assessment period, higher proportions of TMS-receiving patients than of control patients always gave urine samples that tested negative for cocaine. At completion of the assessment period, 69 percent of those treated with TMS had been continuously abstinent from cocaine versus 19 percent of control patients.
Figure. Patients Receiving TMS for Cocaine Addiction Achieve Higher Rates of Abstinence During a 21-day assessment period, higher proportions of TMS-receiving patients than of control patients always gave urine samples that tested negative for cocaine. At completion of the assessment period, 69 percent of those treated with TMS had been continuously abstinent from cocaine versus 19 percent of control patients.
- Text Description of Graphic
-
This line graph compares the proportions of patients with consistently negative drug screens over the 21-day study and demonstrates that patients receiving TMS consistently achieved higher rates of abstinence. All TMS patients were abstinent through day 4. The proportion of abstinent TMS patients fell slowly to 0.9 by day 6, to 0.8 by day 10, to 0.75 by day 14, and to 0.7 by day 18, where it remained through day 21. All control patients were abstinent through day 2, but the proportion of abstinent control patients quickly dropped to 0.5 by day 4, to 0.3 by day 6, to 0.25 by day 10, and to 0.2 by day 14, where it remained through day 21.
A new pilot trial sets the stage for testing the hypothesis definitively in a large-scale placebo-controlled clinical trial. In the trial, Dr. Antonello Bonci of NIDA’s Intramural Research Program, Dr. Alberto Terraneo, Dr. Luigi Gallimberti and colleagues in Italy and the United States, administered a 29-day course of TMS to 16 patients in an outpatient clinic in Padua, Italy. Of the 16, 11 (69 percent) produced 6 cocaine-negative urine samples, and no positive samples, during a 21-day assessment period that started on treatment day 9 (to allow cocaine that the patients had taken before the study to clear their systems) (see Figure). Among a comparison group of 16 patients who received only medications to control symptoms of depression, anxiety, and insomnia, only 3 (19 percent) made it through the assessment period without using cocaine. The TMS-treated patients also reported less craving for cocaine.
In a second phase of the trial, the researchers administered TMS to 10 patients from the original comparison group, 8 of whom had used cocaine during the first phase. Of the 10, 7 (70 percent) then were followed for 63 days post-TMS and achieved abstinence—an outcome nearly identical to that of the patients who received TMS in the first phase.
The researchers have maintained contact with most of the patients in the study. Dr. Bonci says, “While this observation is not part of a rigorous clinical trial follow-up, and should be taken cautiously, the majority of patients who achieved abstinence during the stimulation pilot protocol report that they have maintained that abstinence for more than 2 years. During that time, some patients have requested additional TMS therapy once a week, twice a month, or monthly, and patients can always request additional therapy if they experience cravings. Others report that they have maintained abstinence without additional TMS after the initial set of treatments.”
Aiming and Tuning the Machine
Dr. Terraneo and colleagues’ protocol focuses the TMS electromagnetic field on the left dorsolateral region of the patients’ PFC. Dr. Bonci explains, “This region is accessible and is involved in a number of addiction processes.” In particular, it has been strongly associated with drug craving. In contrast, he adds, “Stimulating the right side can cause anxiety or discomfort in some patients.” (See “A Case for Studying Brain Asymmetry in Drug Use”).
The researchers set the TMS machine to emit magnetic pulses with a frequency of 15 Hz and an amplitude based on each patient’s baseline neuronal responsiveness. The treatment schedule was designed to induce enduring, rather than brief, increases in neuronal responsiveness. Patients underwent TMS on 5 consecutive days during the first study week, then once during each of the remaining 3 study weeks. Each session lasted 13 minutes, during which the patient’s brain was exposed to 2,400 pulses.
Dr. Bonci emphasizes the safety of TMS: “Properly administered, TMS is very safe. The magnetic pulses are much weaker than those generated in an MRI.” Some patients have experienced headaches or pain at the site of stimulation in the first couple of sessions, but, these adverse effects are generally mild and temporary. Dr. Bonci says, “Few medications have such mild side effects.”
The researchers are planning a larger trial with a more rigorous design, which will address some considerations that limit the interpretation of this pilot trial. Because patients’ responses in the pilot trial may have been influenced by knowing whether they were getting TMS or medication, all patients in the new trial will receive either active TMS or sham TMS without knowing which. The new trial will also examine the possibility that TMS helped participants in the pilot trial abstain from cocaine by reducing depression that is experienced by many cocaine users. Dr. Bonci says, “This region [the dorsolateral PFC] has been a TMS target for the treatment of depression for many years.”
“Most likely, TMS should be coupled with behavioral interventions and medication. I would expect a beneficial synergistic effect. Medication may be particularly necessary for difficult cases when TMS alone is not sufficient,” Dr. Bonci adds.
Dr. Harold Gordon, of NIDA’s Epidemiology Research Branch, emphasizes the potential clinical advantages of TMS. “A nonpharmaceutical treatment for addiction would be not only cost-effective but patient-friendly in terms of both compliance and convenience.”
Source:
Terraneo, A.; Leggio, L.; Saladini, M. et al. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study. European Neuropsychopharmacology: 2016. 26(1):37-44. Epub 2015 Dec 4. PMD 26655188. Full text
