This research:
- Showed that high-frequency electrical stimulation of neurons deep in the brain can reduce rats’ relapse-like behavior and motivation to take heroin.
- Strengthens hope that deep brain stimulation might offer a new treatment alternative for opioid addiction, particularly for patients who have not benefited from other treatments.
Researchers are evaluating electrical stimulation of subcortical structures as a possible treatment for a number of neurological and psychiatric disorders, including Parkinson’s disease and severe depression. In small clinical trials, high-frequency electrical stimulation of the subthalamic nucleus (STN HFS) has reduced obsessive-compulsive disorder and other compulsive behaviors. Those findings suggest that STN HFS might help people with addiction overcome urges to take drugs.
To explore that possibility, Drs. Carrie L. Wade and Oliver George and colleagues at the Scripps Research Institute administered STN HFS to rats while exposing the animals to experimental protocols that elicit behaviors that are analogous to human opioid use. Their results suggest that STN HFS may:
- Reduce heroin’s reinforcing effect: Rats pressed a lever to self-administer heroin less often when receiving STN HFS than in an untreated condition. The reduction in lever pressing resulted in lower heroin intake when receiving STN HFS, and indicates that the rats experienced heroin’s reinforcing effect less strongly when receiving STN HFS (see Figure 1A).
- Prevent the rapid relapse to heavy opioid use that typically occurs when a person is reintroduced to the drug after a period of abstinence: Rats that received STN HFS escalated heroin self-administration less rapidly than control animals after a period without access to the drug (see Figure 1B).
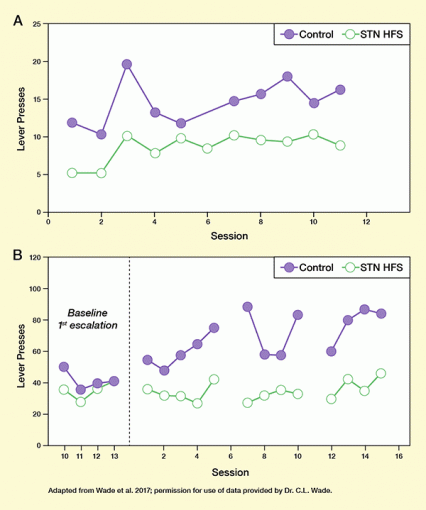 Figure 1. In Rats, STN HFS Reduces Initial Heroin Use and Re-Escalation After Abstinence (Panel A) Given free access to heroin, STN HFS-treated rats self-administered significantly less of the drug than untreated animals. (Panel B) Rats were allowed to self-administer heroin for 10 hours daily for 14 days before undergoing 2 weeks of abstinence. They were then divided into two groups (STN HFS and control) and given access to heroin again in 13 sessions. Sessions 5 and 9 were each followed by 2-day breaks from both heroin and STN HFS. In sessions 10 to 13, the STN HFS-treated animals self-administered significantly less heroin than the control animals, indicating that STN HFS can check the rapid relapse to heavy heroin use that typically occurs when the drug is reintroduced after a period of abstinence.
Figure 1. In Rats, STN HFS Reduces Initial Heroin Use and Re-Escalation After Abstinence (Panel A) Given free access to heroin, STN HFS-treated rats self-administered significantly less of the drug than untreated animals. (Panel B) Rats were allowed to self-administer heroin for 10 hours daily for 14 days before undergoing 2 weeks of abstinence. They were then divided into two groups (STN HFS and control) and given access to heroin again in 13 sessions. Sessions 5 and 9 were each followed by 2-day breaks from both heroin and STN HFS. In sessions 10 to 13, the STN HFS-treated animals self-administered significantly less heroin than the control animals, indicating that STN HFS can check the rapid relapse to heavy heroin use that typically occurs when the drug is reintroduced after a period of abstinence.
- Text Description of Figure 1
-
The figure shows two curves illustrating how high-frequency stimulation of the subthalamic nucleus (STN HFS) reduces initial heroin use and re-escalation after abstinence in rats. Top panel: The graphs show the number of lever press in rats who had free access to heroin during test sessions by pressing a lever. The horizontal (x-) axis shows the number of test session from 0 to 12, the vertical (y-) axis shows the number of lever presses from 0 to 25. The green curve shows the results of animals treated with STN HFS, the purple curve shows the results of control animals, with each filled or empty circle representing one test session. In the STN-HFS animals, the number of lever presses was approximately 5, 5, 10, 8, 10, 9, 10, 9, 9, 10, and 8, respectively, for the test sessions. In the control animals, the numbers were approximately 12, 10, 20, 13, 12, 15, 16, 18, 14, and 17, respectively. At all timepoints, STN HFS-treated rats had fewer lever presses and thus self-administered significantly less heroin than control animals. Bottom panel: The graphs show self-administration of heroin indicated by lever presses in rats after a period of abstinence following heroin use. The x-axis shows the number of sessions. The four timepoints on the left marked “baseline first escalation” represent the last four (10-13) of a series of test sessions in which the animals could freely administer heroin, before undergoing 2 weeks of abstinence. The right part of the figure shows the sessions following the abstinence from session 1 to 16. The y-axis shows the number of lever presses to self-administer heroin from 0 to 120. Rats treated with STN HFS are indicated by the green curve, control animals by the purple curve. During the four sessions of the baseline 1. escalation, the number of lever presses for the animals subsequently treated with STN HFS was approximately 35, 25, 35, and 40, and for the control animals was approximately 50, 35, 40, and 40, indicating comparable self-administration of heroin. The dashed vertical line represents the 2-week abstinence period. Following the abstinence period, the animals had heroin access for 5 sessions with or without STN HFS, followed by 2 days without heroin access and STN HFS, then another 4 sessions of heroin access with or without STN HFS, 2 days without heroin access and STN HFS, and finally another four sessions with heroin access with or without STN HFS. During the first five sessions of heroin access after the 2-week abstinence, STN HFS-treated animals had approximately 35, 30, 30, 25, and 40 lever presses, whereas control animals had approximately 55, 50, 55, 60, and 75 lever presses. During the second set of four sessions with heroin access, STN HFS-treated animals had approximately 25, 30, 35, and 30 lever presses, and control animals had approximately 100, 60, 60, and 90 lever presses. In the last set of four sessions with heroin access, STN HFS-treated animals had approximately 30, 45, 35, and 50 lever presses, and control animals had approximately 60, 85, 95, and 90 lever presses. This indicates that STN HFS can check the rapid relapse to heavy heroin use after a period of abstinence.
Dr. Wade says, “We conclude that deep brain electrical stimulation (DBS) may be an effective strategy for treating people with severe opioid use disorder, particularly patients for whom behavioral and pharmacological treatments have failed.” The technique’s invasiveness will make it less apt, however, for use in patients with less intractable addiction. DBS electrical pulses are administered through micro-electrodes that are surgically placed in the brain. Moreover, multiple DBS sessions might be required to maintain a successful outcome.
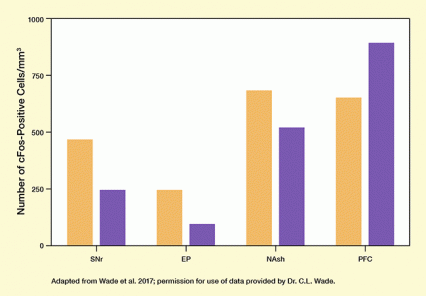 Figure 2. STN HFS Alters Brain Activity in Rats Brain activity was estimated by the levels of cFos, which reflect neural activation, in brain areas that receive signals from the STN. Rats that were treated with STN HFS (purple bars) had lower levels than control animals (orange bars) in three brain regions associated with addictive/compulsive behaviors, but not in a region associated with control over impulsive behaviors. SNr=substantia nigra reticulata; EP=entopeduncular nucleus; NAsh=nucleus accumbens shell; PFC=prefrontal cortex.
Figure 2. STN HFS Alters Brain Activity in Rats Brain activity was estimated by the levels of cFos, which reflect neural activation, in brain areas that receive signals from the STN. Rats that were treated with STN HFS (purple bars) had lower levels than control animals (orange bars) in three brain regions associated with addictive/compulsive behaviors, but not in a region associated with control over impulsive behaviors. SNr=substantia nigra reticulata; EP=entopeduncular nucleus; NAsh=nucleus accumbens shell; PFC=prefrontal cortex.
- Text Description of Figure 2
-
This bar chart illustrates that STN HFS alters brain activity in rats in areas receiving signals from the subthalamic nucleus. The four groups of bars represent analyses in four brain areas. These include the substantia nigra reticulata (SNr), entopeduncular nucleus (EP), and nucleus accumbens shell (NAsh), which are associated with addictive compulsive behaviors, and the prefrontal cortex (PFC), which is associated with control over impulsive behaviors. The vertical (y-) axis shows the number of cells positive for the cFos, which reflects brain activity, from 0 to 1,000 cells/mm3. Control animals are indicated by orange bars, rats treated with STN HFS are indicated by purple bars. In the SNr, the number of cFos-positive cells/mm3 is approximately 450 in control animals and about 250 the STN HFS-treated animals. In the EP, the number of cFos-positive cells/mm3 is about 250 in control animals and about 100 in STN HFS-treated animals. For the NAsh, the number of cFos-positive cells/mm3 is about 700 for control animals and about 500 for STN HFS-treated animals. This shows reduced brain activity in the STN HFS-treated animals than the control animals in these regions. In the PFC, the number of cFos-positive cells/mm3 is about 650 for control animals and about 900 for STN HFS-treated animals, indicating higher brain activity in the STN HFS-treated animals than the control animals.
The mechanisms whereby DBS may produce therapeutic effects remain ill-defined. Drs. Wade and George and colleagues used immunohistochemistry and electrophysiology to assess the effect of STN HFS on neuronal activity in rats. They found that their STN HFS regimen reduced neuronal activity in various brain regions and increased it in others. Activity declined in the nucleus accumbens shell, a region that mediates the reward value of drugs, and increased in the prefrontal cortex, a center of control over impulsive behaviors (see Figure 2).
Dr. Wade says, “These results identify novel targets for DBS and also may facilitate the development of noninvasive brain-stimulation techniques that can target these brain regions.” One such noninvasive technique, transcranial magnetic stimulation, has shown potential as a treatment for cocaine dependence.
Further studies are needed to establish whether DBS will have clinical utility as a treatment for drug dependence and, if so, in what circumstances. Drs. Wade and George note that animal studies such as theirs cannot model all of the various factors that can lead human patients to drop out of treatment or relapse after having achieved abstinence. Nevertheless, says Dr. George, “Although DBS is invasive compared with other forms of treatment, such as medication-assisted treatment, replacement therapy, behavioral therapy, and behavioral modification, it may represent a viable alternative for patients who are at high risk of overdose and for whom other treatments have failed.”
The study was supported by NIH grant DA029821.
Source:
Wade, C.L.; Kallupi, M.; Hernandez, D.O. et al. High-frequency stimulation of the subthalamic nucleus blocks compulsive-like re-escalation of heroin taking in rats. Neuropsychopharmacology 42(9):1850-1859, 2017.
