Role of Research
During these historic times for scientific advancement, NIDA-funded investigators have had another 3 years of exceptional accomplishment. NIDA-supported researchers have made enormous strides toward understanding, preventing, and treating one of our Nation's most serious public health problems-drug use and addiction. Today we know more about the effects of abused drugs on the brain than is known about almost any other aspect of brain function.
Research on drug use and addiction crosses many scientific, social, and cultural boundaries, and it must be transferred from the laboratory to the clinic to the community and back again. For example, our ability to improve the effectiveness of drug use prevention and treatment depends on our understanding the underlying neurobiology of addiction as well as the biological, genetic, social, psychological, and environmental factors that predispose individuals to drug addiction.
The NIDA research portfolio is paying dividends to society in at least two major ways. First, there are the specific accomplishments, some of which are detailed in the following pages, that are generating new insights into drug use and addiction, producing new treatments for drug addiction, developing prevention methods that work, and enabling us to disseminate this information into the clinical community and general public. However, just as important, scientific research and clinical experience have taught us much about what really matters in addiction and where we need to concentrate both our clinical and policy efforts.
One of the best examples of this payoff can be seen in how we have now come to define addiction. Research has shown that differentiating between physical and psychological addiction is outdated. Twenty years of scientific research, coupled with even longer clinical experience, has taught us that focusing on this physical vs. psychological distinction is off the mark, and a distraction from the real issues. From both clinical and policy perspectives, it actually does not matter very much what physical withdrawal symptoms occur. Other aspects of addiction are far more important.
Physical dependence is not that important because, first, even the florid withdrawal symptoms of heroin and alcohol addiction can now be easily managed with appropriate medications. Therefore, their physical withdrawal symptoms should not be at the core of our concerns about these substances.
Second, and more important, many of the most addicting and dangerous drugs do not even produce very severe physical symptoms and withdrawal. Crack cocaine and methamphetamine are clear examples. Both are highly addicting, but stopping their use produces very few physical withdrawal symptoms, certainly nothing like the physical symptoms of alcohol or heroin withdrawal.
What does matter is whether a drug causes what research has now shown convincingly to be the essence of addiction: compulsive drug-seeking behavior and use, even in the face of negative health consequences. These characteristics ultimately matter most to the patient and are where treatment efforts must be directed. These behaviors are also the elements responsible for the massive health and social problems that drug addiction brings in its wake.
To ensure that NIDA-sponsored research continues to provide valuable insights into the nature of addiction, the Institute has continued to work closely with the research, treatment, and health services communities, as well as with other Federal agencies and the States, to develop long-range research plans for several high-priority areas. The following sections of this chapter describe the progress that NIDA has made in addressing these areas of research priority.
Neurobiology, Genetics, and Behavior
All drugs of abuse act in the brain by altering normal biological processes, which in turn cause changes in behavior and thinking. Understanding the relationship between brain mechanisms that underlie behavior and drug-related changes in these mechanisms is critical for developing more effective treatments for drug addiction and its consequences. So, too, is understanding how genetic differences, and environmental influences on these differences, among members of the population can make some people either more or less vulnerable to addiction to both licit and illicit drugs.
During this decade, we have learned much about the neurobiological, genetic, and behavioral aspects of drug addiction. Scientists have identified neural circuits that subsume the actions of every known drug of abuse, and they have specified common pathways that are affected by almost all such drugs. Researchers have also identified and cloned the major receptors for virtually every abused drug, identified their natural ligands, developed methods to directly manipulate receptor systems, and begun linking the neurobiology of these receptors to behaviors seen during drug use and addiction. In addiction, they have elaborated many of the biochemical cascades within the cell that follow receptor activation by drugs. Research also has begun to reveal major differences between the brains of those at risk for addiction and among those without risk factors.
Research also has identified differences between the brains of those at risk for addiction and indicated some common brain elements of addiction, regardless of the substance involved. Genetic studies have been useful in identifying inherited traits that are strongly linked to substance abuse and its risk factors and in suggesting neurobiological and behavioral connections.
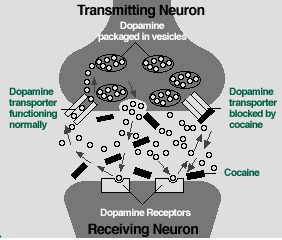 Source: National Institute on Drug AbuseCocaine in the Brain
Source: National Institute on Drug AbuseCocaine in the BrainBelow are descriptions of a few of the most significant research accomplishments since 1995 in the areas of the neurobiological, genetic, and behavioral aspects of addiction.
Cocaine Addiction
During the past 3 years, our knowledge about how cocaine affects the brain has reached a critical mass. For the first time, we have important insights into the amazingly complex neurotransmission system involved in cocaine addiction. These advances have opened new doors for potential therapeutic intervention. For example, neurochemical studies of cocaine's effects on the brain's reward system have teased out some of the details of how this drug interacts with the dopamine system. Repeated self-administration of cocaine appears to be tied to the D1 receptor type, whereas three dopamine receptor types-D1, D2, and D3-are involved in cocaine-seeking behaviors. [8] Another study [9] found that activating the D1 receptor system can suppress cocaine seeking in drug-experienced animals. In contrast, activating the D2 receptor system can trigger cocaine seeking. This makes D1 receptor stimulation a potential target for the development of medications to treat cocaine addiction. In fact, formerly addicted rats pretreated with a chemical known to activate only the D1 receptor system were completely resistant to cocaine's ability to prime or restart drug use.
Until recently, most of what we have known about the relationship between cocaine and dopamine came from animal models. During the past 3 years in particular, imaging studies that used positron emission tomography (PET) and functional magnetic resonance imaging have for the first time shown that dopamine neurotransmitter systems in the human brain respond to cocaine in a manner similar to that seen in laboratory animals. In one study, PET imaging showed that there was a significant relationship between the intensity and duration of a cocaine high and the degree to which cocaine was able to increase the amount of dopamine available in the brain. In this instance, cocaine was seen blocking the dopamine reuptake system that removes dopamine from the synapse between neurons-that keeps more dopamine in the synapse and has the effect of allowing these neurons to propagate nerve signals for a longer period than normal. [10] The same group of investigators also used PET to identify some of the unique properties of cocaine that account for the binge pattern of use exhibited by cocaine addicts. Their studies showed that repeated and frequent activation of the dopamine system by cocaine results in an abnormal state in one of the brain's neural circuits. [11] This altered brain circuit may be responsible for the dramatic difference in brain functioning that exists between the nonaddicted and addicted individual.
Investigators have used PET to see changes in the brain triggered by environmental cues that are associated with past experiences of using drugs and that lead to drug craving, even when no drug is available. The brain regions activated during exposure to the drug-related stimuli were those involved in integrating the emotional and cognitive aspects of memory. As a result, researchers now have the first evidence that the mechanisms of memory processing are as important to the understanding of cocaine craving as the direct effects of the drug on the nervous system.
Although it is clear that the dopamine system is intimately involved in cocaine addiction, as it is with all drugs of abuse, other brain systems must also come into play. Evidence compiled over the past 3 years has shown that the serotonin system is certainly involved in cocaine addiction. Investigators have now shown, for example, that blocking cocaine's effects on the serotonin 5-HT3 receptor prevents the occurrence of tolerance and sensitization to cocaine. Further research on this neurotransmitter system found that continuous cocaine administration decreases the number of 5-HT3 receptors in the nucleus accumbens. Because the nucleus accumbens is thought to be critical in mediating reinforcement processes in general, this decrease may be critical in our understanding of the consequences of cocaine use. [12]
Researchers have also found that mice lacking the gene for another serotonin receptor, known as 5-HT(1b), were more responsive to cocaine and were therefore more susceptible to cocaine addiction than mice having that gene. Further work with these mice showed that they were also more susceptible to the effects of alcohol. Finally, investigators have identified, in the brains of humans, monkeys, and guinea pigs, a new serotonin receptor, named SERTsite2, that binds only cocaine and not other drugs that interact with the serotonin system. This finding suggests that this new receptor may be involved in behaviors caused specifically by cocaine and that this receptor might be a good target for the development of drugs to treat cocaine abuse or addiction.
Additional genetic studies have shown that still other brain neurotransmitter systems are involved in cocaine addiction. This conclusion was determined by using the genetic technique known as "knockouts." Using this method, strains of mice can be developed that lack parts of the serotonin and dopamine systems known to interact directly with cocaine. Even missing these components, specifically the serotonin and dopamine transporters, these mice remained sensitive to the rewarding effects of cocaine. [13] As a result, investigators are now searching for the other neurotransmitter systems involved in cocaine addiction.
Nicotine Addiction
In a landmark study, investigators using a strain of mice lacking a specific region of the nicotine receptor have identified the first neurochemical step in the pathway toward nicotine addiction.[14] Using sophisticated bioengineering tools, the researchers developed a strain of mice lacking the beta-2 subunit, one of the 10 proteins that form the brain's nicotine receptor. These mice fail to self-administer nicotine, implying that without the beta-2 subunit the mice do not experience the positive reinforcing properties of nicotine. Importantly, in this study both the normal and the genetically altered mice did self-administer cocaine, indicating that even without this beta-2 subunit, the brain reward pathway thought to be common to all addictions remains intact even though nicotine loses its effect. In addition, nicotine injections given to these mice did not increase levels of the neurotransmitter dopamine, something that is typically seen following nicotine administration.
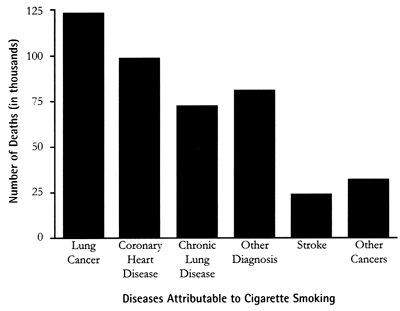 Source: Centers for Disease Control and Prevention430,000 Annual Deaths Attribute to Cigarette Smoking - United States, 1990 - 1994
Source: Centers for Disease Control and Prevention430,000 Annual Deaths Attribute to Cigarette Smoking - United States, 1990 - 1994PET imaging studies with human smokers and nonsmokers have found that cigarette smoking was associated with a significant reduction in brain levels of the enzyme monoamine oxidase-A (MAO-A). These findings are similar to earlier experiments that showed a reduction of brain monoamine oxidase-B levels in smokers. Because drugs that inhibit MAO-A are effective antidepressants, these results suggest that MAO-A inhibition should be considered as a potential contributing factor in the high rate of smoking among depressed individuals as well as in the development of more effective strategies for smoking cessation. [15]
Other avenues of research are also elucidating brain changes following long-term smoking. For example, postmortem studies comparing the brains of human smokers and nonsmokers found that lifelong smokers had far more nicotine receptors than nonsmokers did in key brain regions. Furthermore, the amount of increase correlated with the degree of smoking as measured by the average number of packs smoked per day, a finding that agrees with those from animal studies. One important finding from this study was that nicotine receptor levels fell to normal in lifetime smokers who had quit at least 2 months prior to death, suggesting that nicotine tolerance and addiction produce changes in brain neurochemistry that may be reversible with time.
Investigators have also found that there are dramatic changes in the brain's pleasure circuits during withdrawal from chronic nicotine use that rival the magnitude and duration of similar changes observed during withdrawal from other abused drugs, such as cocaine, opiates, amphetamines, and alcohol. These changes were seen as decreases in the sensitivity of the brains of laboratory rats to pleasurable stimulation after nicotine administration was abruptly stopped. These changes also lasted several days and may correspond to the anxiety and depression experienced by humans for several days after quitting smoking "cold turkey." The results of this research indicate that we have a good animal model in which to study the neurobiology of nicotine abstinence and thus to assist with the development of behavioral and pharmacological treatments for nicotine addiction. [16]
However, new evidence suggests that nicotine is not the only drug in tobacco responsible for the addictive potential of cigarette smoking. In a recent experiment, investigators compared the mood effects in humans produced by a standard 1-mg nicotine cigarette versus a cigarette with all nicotine removed. As other studies have shown, subjects reported feeling calmer and less irritable after smoking the cigarette containing nicotine. However, subjects reported many positive subjective effects after smoking the cigarette lacking nicotine, effects that were comparable in magnitude to the nicotine cigarette. These results raise the possibility that the airway sensory effects of cigarette smoke alone contribute to the positive subjective effects of smoking. These results further suggest that airway sensory replacement therapy may be useful for smoking cessation. [17]
Opiate Addiction
It has been known since the 1970s that the body has a natural opioid system that acts in much the same way as opiate drugs like heroin and morphine. Not only are there receptors throughout the body that respond to opiate substances, but the body produces natural opioids that are released during strenuous exercise and in response to stress or pain. These natural substances are involved in the body's control of pain reactions and play a role in many other behaviors, such as the experience of pleasure. Now researchers have discovered that the body's natural opioid system is far more important and has broader effects than ever thought. In addition to being essential to responses to pain and for the euphoria from such drugs as morphine, codeine, and heroin, the mu opioid receptor, the cellular target of these drugs, also appears to be in-volved in regulating the immune and reproductive systems. Mice without the gene for the mu opioid receptor show reduced sexual and reproductive function and altered immune systems. These observations in the mouse model track well with anecdotal accounts that humans using heroin and morphine experience both reduced immune function and reduced sex drive. Not only do opiates affect mood states and produce addiction, but it also appears that their effects on natural opioid receptors lead to changes in the ability of the affected mice to reproduce and to resist infection and disease.
Another important finding regarding opiate addiction is the discovery of a neuronal system in the brain that modulates and opposes the action of the brain's opioid system. The discovery of the actions of orphanin FQ (OFQ), a natural brain chemical, could provide the foundation for the development of effective painkilling drugs without some of their negative side effects.[18] Indeed, new evidence that OFQ and its receptor may play a role in opioid function will lead to new research exploring the balance or homeostasis of the opioid system to determine whether an imbalance or overactivity in one part of the brain usually leads to a compensatory change that counterbalances it. These findings raise interesting possibilities for developing therapeutic agents that act to compensate for the effects of the OFQ receptor because blocking it may decrease the irritability, anxiety, and nausea characteristic of heroin withdrawal.
Investigators have also uncovered the first direct evidence that long-term, chronic opiate exposure is associated with structural changes in both the size and shape of specific neurons in the brain. In previous animal studies, scientists have shown that chronic exposure to opiates causes dramatic biochemical changes in brain cells that could contribute to the intense craving for these drugs during withdrawal states. The physical changes seen in this new study occur in the dopamine system within a specific region of the brain known as the ventral tegmental area. This brain area is activated during the use or self-administration of virtually all drugs of abuse. The next step is to determine exactly what these changes mean and how they might be addressed in the development of new medications for the treatment of the long-term consequences of opiate use. Given the prominence of these changes, effective treatment of addictive disorders for most people would have to include treatments to undo or compensate for these biological changes.
As is the case with cocaine abuse, slight variations in key metabolic enzymes can afford some protection against opiate addiction. Oral opiates, such as codeine and oxycodone, are metabolized by an enzyme called cytochrome CYP2D6 to metabolites of increased activity-morphine and oxymorphone, respectively. Between 4 and 10 percent of Caucasians lack this enzyme; they would be considered poor metabolizers. A study of individuals addicted to opiates found that none of them was a poor metabolizer. This underrepresentation of poor metabolizers in people dependent on oral opiates suggests that the CYP2D6 defective genotype is a pharmacogenetic protection factor for oral opiate dependence. [19]
Marijuana Abuse
Given that marijuana is the Nation's most commonly abused illicit drug, understanding its mechanism of action will be a critical step in developing new therapies for preventing and treating marijuana use. For example, researchers now know that genetic factors may play a larger role than originally thought in how an individual responds to a drug such as marijuana.
Investigators have now determined that heredity strongly influences whether an individual has positive or negative sensations after smoking marijuana. This study demonstrated that identical male twins were more likely than nonidentical male twins to report similar responses to marijuana use, indicating a genetic basis for their sensations. The finding that genetic factors contribute to how an individual feels after using marijuana opens new avenues for prevention and treatment research; it further emphasizes that drug use and addiction are not simply social problems but are health issues affected by an individual's biological state. Environmental factors may lead to an individual's experimenting with a drug, but heredity appears to hold a key to whether an individual will continue to use or abuse the drug.
One study of long-term use of marijuana showed that marijuana produces changes in the brain that are similar to those seen after long-term use of other major drugs of abuse, such as cocaine, heroin, and alcohol. Moreover, these changes may increase a user's vulnerability to addiction to other abusable drugs by "priming" the brain to be more easily changed by drugs. Importantly, the study also showed that the specific brain areas that were activated during marijuana withdrawal are quite active during withdrawal from other drugs of abuse and play a key role in stress responses in general.
Another study of long-term marijuana users found that many individuals continued to smoke heavily into middle adulthood because they felt that marijuana relieved unpleasant feeling states, such as anxiety or depression. A subsequent review of the medical literature found a series of cases in which marijuana was used because it reportedly produced a direct antidepressant effect in those who had mood disorder. These observations argue the point that many patients may use marijuana to "self-medicate" depressive symptoms.
Exploring the Links Between Drug Abuse and Other Behavioral Disorders
Numerous epidemiological studies have shown that drug use disorders are frequently associated with mental disorders. For example, data from the Epidemiologic Catchment Area study showed that 53 percent of individuals who have a lifetime diagnosis of a drug use disorder also have a lifetime diagnosis of a mental disorder. Approximately two-thirds of individuals with a cocaine use or opiate use disorder have, at some point in their lives, had a mental disorder. For those with a lifetime diagnosis of any mental disorder, 15 percent have had a drug use disorder. Twenty-eight percent of people with schizophrenia and 42 percent of those diagnosed with antisocial personality disorder (ASPD) have had a drug use disorder.
With the strength of the association between drug use and mental health disorders well established, investigators have sought to better understand how other behavioral disorders might contribute to substance use and addiction. One group has been studying the prevalence of attention deficit/hyperactivity disorder (ADHD) in a cross-section sample of adults seeking substance abuse treatment. ADHD is considered to be a genetic and neurobiological disorder with a 5-percent prevalence rate in all U.S. children. In this study, 24 percent of adult substance users met the clinical criteria for ADHD, both as a child and as an adult. The study also found that substance users with ADHD were more likely to have conduct disorder and ASPD and more motor vehicle accidents than substance users without ADHD. Although the use of specific types of drugs was not significantly different between the groups of substance users with and without ADHD, female substance users with ADHD had an increased number of treatments for alcohol abuse and dependence. [20]
In a prospective longitudinal study of substance-using adolescents who met the clinical criteria for conduct disorder, investigators found that 61 percent of the study group met the clinical criteria for ASPD 4 years after entering the study. Pretreatment clinical characteristics that predicted posttreatment ASPD included deviant behavior at or before age 10, greater diversity of deviant behaviors independent of substance use during childhood and early adolescence, and more extensive drug, but not alcohol, use during the 30 days before admission to the program. These findings suggest a poorer prognosis for adolescents when conduct disorder is diagnosed independent of drug use, whereas more favorable drug treatment outcomes might be achieved with adolescents whose pretreatment diagnosis of conduct disorder occurred primarily in the context of or subsequent to their use of illicit substances. [21]
In the case of marijuana use, investigators have found that conduct disorder, ADHD, and major depression correlate with dependence in a population of adolescents referred for substance abuse problems. The subjects reported that progression from first use of marijuana to regular use was as rapid as tobacco progression and more rapid than that of alcohol, suggesting that marijuana is a potent reinforcer. Data from this study indicate that for adolescents with conduct problems marijuana use can be particularly hazardous. The drug potently reinforces marijuana smoking, producing both dependence and withdrawal.
Indications of just how ADHD might be tied to drug use are coming from several completed and ongoing genetic studies involving the dopamine system. One group of investigators has found, for example, that individuals who possess rarer variants of three different genes for dopamine system components had a significantly higher incidence of ADHD symptoms. Individuals with two of the variants had fewer symptoms but still met clinical criteria for this behavioral disorder, whereas those with one variant gene were considered borderline for ADHD. These findings, along with several others nearing completion, contribute to the notion of a genetic basis for a syndrome, characterized by a deficiency in the brain's reward systems, that consists of addictive, impulsive, and compulsive behavior and personality disorders. [22]
