Details
Contact
United States
Meeting Summary
NIDA's Neuroscience Consortium organized the 9th annual Frontiers in Addiction Research Mini-Convention held at the 2010 Society for Neuroscience Meeting, November 12, 2010 in San Diego, CA. The mini-convention included sessions on the role of nicotinic receptors in the habenula in mediating addiction; using model organisms to discover unanticipated pathways to addiction; a fresh look at dopamine release and uptake; and connectivity of the human brain and its disruption by drugs of abuse.
Session Summaries:
- The Role of Nicotinic Receptors in the Habenula in Mediating Addiction
-
Overview
Numerous genome-wide association studies have found that SNPs in the alpha3-beta4-alpha5 nicotinic receptor cluster on chromosome 15q24 are associated with nicotine dependence. Although it is reasonable to presume that this association is mediated by the reward system centered on the mesolimbic dopamine pathway, recent work suggests that the effects of alpha3-beta4-alpha5 nicotinic receptors on drug-seeking behavior are mediated, instead, by actions centered on the habenula and interpeduncular nucleus. In this symposium, speakers presented the unique neurobiological properties and anatomy of this system, and its relation to drug abuse and addiction. When combined with the results of previous genome-wide association studies, the presentations provided new insights into the biological basis of individual vulnerability to drug addiction.
Chair:
Dr. Jonathan Pollack
National Institute on Drug AbuseRepresentation of Negative and Positive Motivational Values in the Monkey Lateral Habenula
Okihide Hikosaka, M.D., Ph.D.
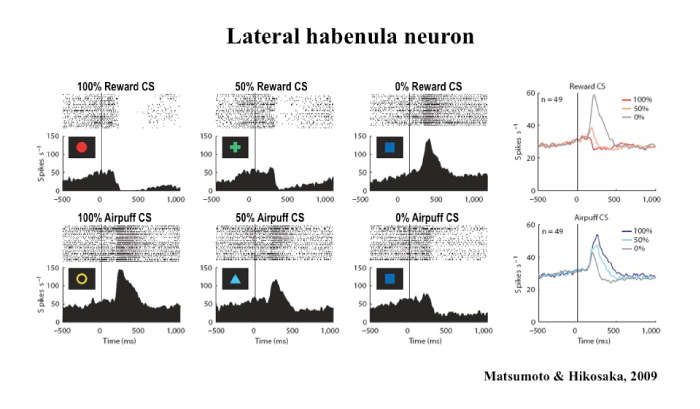
This presentation focuses on the relationship between the lateral habenula and the basal ganglia, a group of nuclei in the brain associated with a variety of functions, including voluntary muscle movement, reasoning, emotional responses, and procedural learning related to habits. Many studies suggest that the basal ganglia facilitate behaviors leading to larger rewards and suppress behaviors leading to smaller rewards. A key factor in this behavior change is the release of dopamine in the basal ganglia, as most dopamine neurons are excited by a larger-than-expected reward (or its predictor) and inhibited by a smaller-than-expected reward (or its predictor).
 Download the Presentation (PDF, 901Kb)
Download the Presentation (PDF, 901Kb)However, previous studies have not determined how or why dopamine affects learning and causes the brain to predict the anticipated amount of reward accurately. Dr. Hikosaka and his team of researchers recently learned that the lateral habenula, a small area in the thalamus, inhibits dopamine neurons in the basal ganglia and thereby controls motor and cognitive behaviors. A majority of lateral habenula neurons were excited by a visual stimulus predicting a small reward and inhibited by a stimulus predicting a large reward, a pattern opposite to that seen in dopamine neurons. Microstimulation of the lateral habenula caused a clear inhibition in most of the dopamine neurons. Therefore, lateral habenula neurons send reward-related signals to dopamine neurons while converting it from negative reward signals to positive reward signals. Researchers also found that aversive events (or their predictors) excite lateral habenula neurons and inhibit a group of dopamine neurons. These results demonstrate that the basal ganglia are under critical control of neural circuits outside the basal ganglia involving the lateral habenula.
The Habenula and Nicotine Withdrawal: From Mouse to Human
Ramiro Salas, Ph.D.
 Download the Presentation (PDF, 3.9MB)
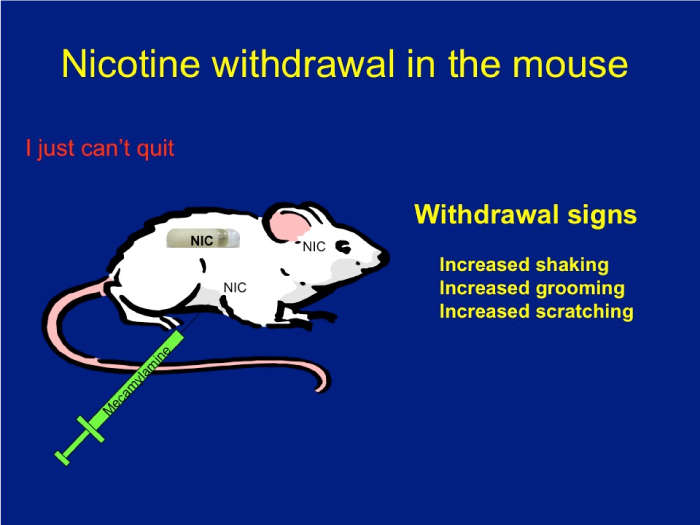
Download the Presentation (PDF, 3.9MB)Nicotine has an addictive effect on the brain because it binds nicotinic acetylcholine receptors. These receptors are ion channels modulated by nicotine. The role of each receptor in normal physiological and pathological states has been extensively debated. Using mice that lack different subunits of the nicotinic receptors, Dr. Salas and his research team were able to define a group of subunits—alpha2, alpha5, and beta4—as directly influencing nicotine withdrawal.
To illustrate and analyze symptoms of withdrawal, mice were delivered saline or nicotine continuously for 2 weeks. They were then injected with mecamylamine, a chemical that blocks nicotinic receptors, which caused withdrawal signs such as scratching, grooming, and shaking. These signs were monitored for 20 minutes. Using knockout mice that lack each subunit, the research team found that nicotinic receptors made up of the alpha2, alpha5, and beta4 subunits play a critical role in nicotine withdrawal in mice. Also, blocking nicotinic activity only in the habenula or its main target, the interpeduncular nucleus (but not in other areas), results in withdrawal signs in nicotine-treated mice.
Since these subunits are expressed mainly in the habenula, the researchers decided to study habenular activity in humans using functional Magnetic Resonance Imaging (fMRI), a type of specialized MRI scan that measures changes in blood flow related to neural activity in the brain. Using this technology, they demonstrated that when the brain experiences a smaller-than-expected reward, the habenula is activated. Volunteers viewed cues on a computer screen and received small squirts of sweet juice in their mouth to experience reward. Later, the cue-juice timing was manipulated to produce disappointment (juice expected but not delivered). The fMRI illustrated an activated habenula when a reward was expected but not delivered. This insight will allow researchers to study habenular activity during smoking and tobacco abstinence. The researchers hypothesize that habenular hyperactivation may be the mechanism that triggers tobacco withdrawal in humans, which is in agreement with enhanced negative feelings during attempts to quit smoking.
- Using Model Organisms to Discover Unanticipated Pathways to Addiction
-
Overview
Genetic studies in both humans and model organisms (such as worm, fly, zebrafish, and mouse) are leading to the identification of new and previously unsuspected genes involved in addictive processes. This symposium highlighted the use of model genetic organisms to identify novel genes or gene variants involved in addictive processes. Dr. Chesler described a mouse systems genetics approach to identify genes involved in a suite of substance abuse-relevant behavioral phenotypes. Dr. Heberlein described her work in fruit flies to identify a novel receptor protein kinase pathway involved in cocaine sensitivity. All speakers discussed strategies to translate their findings into new diagnostic or therapeutic tools for substance abuse.
Chair:
Dr. John Satterlee
National Institute on Drug AbuseSystems Genetics Identification of New Addiction Genes in Mice Using High-Throughput Behavioral Phenotyping
Elissa J. Chesler, Ph.D.
 Download the Presentation (PDF, 467Kb)
Download the Presentation (PDF, 467Kb)Genetic variation determines differences in behavior and brain function. It can also help identify and characterize molecular processes associated with the brain and behavior, or neurobehavioral phenomena. Systems genetics is the practice of simultaneously examining the effect of naturally occurring genetic polymorphisms (differences in DNA sequence among individuals) on networks of molecular and phenotypic processes. Dr. Chesler and her team of researchers used this approach to identify genes and gene networks that alter behaviors associated with drug- and alcohol-related disorders.
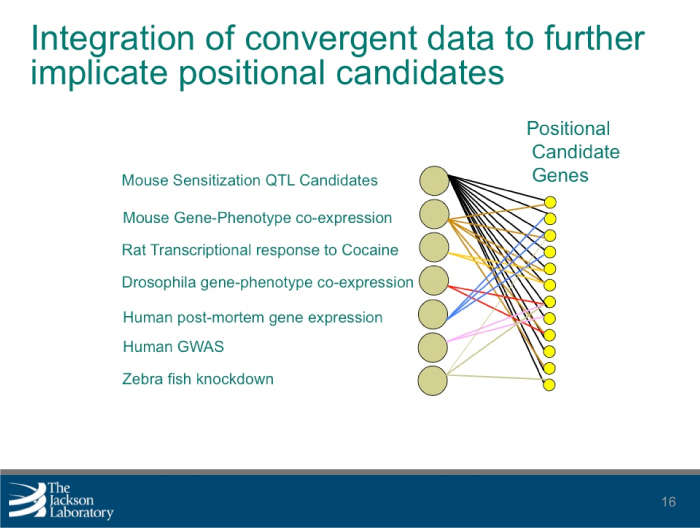
The research team used mice to identify multiple substance abuse-related behaviors, which resulted in more than 250 observable characteristics or traits (phenotypic values) from 49 tests. These values, which included cocaine response and sensitization, morphine response, and naloxone-precipitated morphine withdrawal, were then combined with existing molecular and genetic data to perform genetic mapping. This resulted in the detection of 25 significant quantitative trait loci, or regions containing one or more genes responsible for variation. An additional 70 regions were identified for the phenotypes investigated and analysis revealed multiple phenotypic factors, which were compared with additional measures of behavior obtained in the same population.
By combining the behavioral data with gene expression data from multiple tissues, the research team identified positional candidate genes and molecules associated with changes in behavior. The team is further refining these candidate gene lists by combining related gene-centered data. Researchers can use the polymorphisms, or distinct characteristics that exist in the same species, to identify how behavior relates to underlying molecular processes. By doing so, the discovery of pathways and mechanisms that illustrate the behavioral tendency to abuse drugs can be accelerated.
Anaplastic Lymphoma Kinase is a Transcriptional Target of LMO4 and Estrogen that Controls Cocaine-Induced Behavioral Plasticity
Ulrike Heberlein, Ph.D.
 Download the Presentation (PDF, 467Kb)
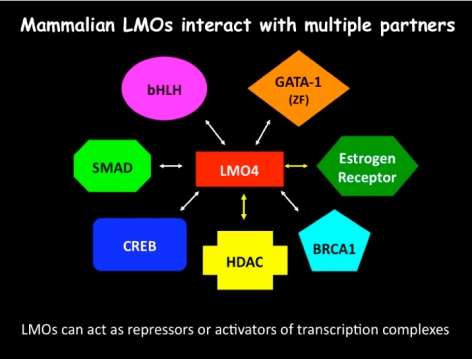
Download the Presentation (PDF, 467Kb)This presentation described a new molecular system in two species that is important for the response to cocaine. Dr. Heberlein and his research team showed that the fruit fly (Drosophila) protein dLMO and the related mouse protein LMO4 control the effects of cocaine on the brain and behavior. Both proteins are involved in modifying the transfer of genetic information from DNA to messenger RNA, thus regulating the expression of genes in the brain. The nucleus accumbens, a brain region implicated in drug addiction in mammals (including humans), is strongly affected by cocaine. The research team found that the activity of LMO4 in the nucleus accumbens is important for behaviors in mice related to long-term exposure to cocaine. They have identified genes that are regulated by dLMO in fruit flies. Among these is the gene encoding the receptor tyrosine kinase dALK, whose mammalian homolog, anaplastic lymphoma kinase, has been implicated in many cancers as well as depression-related behaviors.
These studies suggest that identifying genes in the brain controlled by dLMO in fruit flies and LMO4 in the mouse will be important for finding new approaches to treat cocaine addiction in humans.
- A Fresh Look at Dopamine Release and Uptake
-
Overview
Cutting edge findings that challenge "what we learned in school" about dopamine (DA) release were presented. New approaches, such as direct observation of DA uptake and release from individual presynaptic terminals, and new findings, including glutamate co-transmission in DA neurons, are contributing greatly to our understanding of the coding of incentive salience of conditioned stimuli and the mechanisms underlying enduring drug-seeking behaviors. The focus on presynaptic regulation of DA release is also relevant to schizophrenia, attention-deficit hyperactivity disorder, and Parkinson's disease.
Co-Chairs:
Dr. Jerry Frankenheimer
National Institute on Drug AbuseDr. Nancy Pilotte
National Institute on Drug AbuseThe Dopamine Transporter: More Exciting than Housekeeping
Susan Ingram-Osborn, Ph.D.
 Download the Presentation (PDF, 1MB)
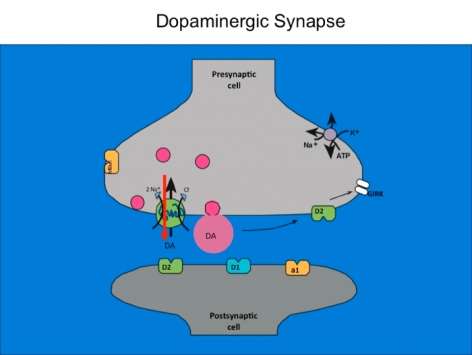
Download the Presentation (PDF, 1MB)The dopamine transporter is commonly known for halting dopamine actions through re-uptake of the released dopamine into the presynaptic cell. Less well known is the fact that dopamine and other enzyme-influenced substances serve as an opening to an ion channel that is selectively permeable to chloride. Dr. Ingram-Osborn and her research team are currently exploring how this channel alters dopamine neuron activity. Transporter-controlled electrical currents represent unexplored avenues for cellular mechanisms of psychostimulant action and potential targets for novel treatments of drug addiction.
In order to measure how dopamine neuron activity is modulated, the research team used whole-cell patch-clamp recordings from rat dopamine neurons in either culture or brain-slice preparations to characterize the dopamine transporter-mediated current. They found that dopamine transporter substrates such as dopamine and amphetamine gate a chloride current that reverses close to the resting membrane potential of the dopamine neurons. Activation of this current can increase the excitability of dopamine neurons, suggesting that activation of that dopamine transporter may enhance the release of dopamine. Recent data have demonstrated that the dose-response curve for amphetamine activation of the dopamine transporter-mediated chloride current is U-shaped, suggesting that amphetamine has different effects at low and high concentrations. Experiments designed to better understand chloride regulation in dopamine neurons are ongoing.
Optical Means to Detect Synaptic Personality
David Sulzer, Ph.D.
Dopamine signaling via basal ganglia synapses is required for learning and for the selection of appropriate behavior. To determine how dopamine selects particular basal ganglia synapses, Dr. Sulzer and his research team developed optical techniques that measure activity of individual presynaptic areas. Synapses were analyzed in sections of the central nervous systems of mice, and synaptic pathways were stimulated extracellularly. The team's findings indicate that, at low frequency (1 Hz) stimulation, three types of synapses maintain normal distributions of activity. By 10 Hz, each synaptic pathway exhibits a fraction of terminals with substantially higher activity. Interestingly, methamphetamine administered in vivo creates permanent changes in synaptic selection, which the team members label chronic presynaptic depression and paradoxical presynaptic potentiation. These changes may last for the lifetime of the animal and are renormalized by amphetamine readministration and involve dopamine effects on cholinergic signaling.
The ability to measure presynaptic activity in populations of individual terminals indicates an unsuspected range of activity within classical projections. This activity is dependent on neuronal firing patterns, so that simultaneous activation of neural pathways causes particular synapses to be selected. Dopamine contributes to this selection and can either dampen or enhance activity of particular presynaptic terminals in a frequency-dependent manner, depending on involvement of particular receptors. Together, these findings may explain how the union of two or more neural pathways alters synaptic strength such that appropriate behaviors are learned and selected. The ability of an addictive drug that releases dopamine to produce the longest changes in synaptic plasticity yet reported may underlie lifelong alterations involved in dependency.
- Connectivity of the Human Brain and Its Disruption by Drugs of Abuse
-
Overview
Functional magnetic resonance imaging (fMRI) is advancing beyond the identification of task-activated brain regions, and is now being used to characterize the structure and dynamic function of inter-regional brain networks. Aberrant connectivity patterns have recently been shown to be characteristic of neuropsychiatric disorders including drug abuse and are also related to impaired performance on neurocognitive tasks.
Chair:
Dr. James Bjork
National Institute on Drug AbuseThe Restless Brain
Marcus E. Raichle, M.D.
 Presentation not available
Presentation not availableTraditionally, studies of brain function have focused on task-evoked responses. By their very nature, such experiments encourage a spontaneous view of brain function. The alternative possibility is that brain functions are mainly built-in, involving information processing for interpreting, responding to, and predicting environmental demands. Judged from the perspective of brain energy consumption, it is this alternative perspective that best captures the essence of brain function. Understanding the nature of this ongoing and basic activity requires integrating knowledge from cognitive and systems neuroscience with cellular and molecular neuroscience.
Delineating Intrinsic Connectivity Networks for Emotional Salience Processing and Executive Control
Michael Greicius, M.D.
 Download the Presentation (PDF, 2.2MB)
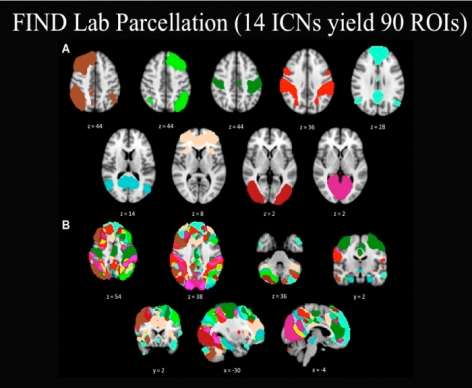
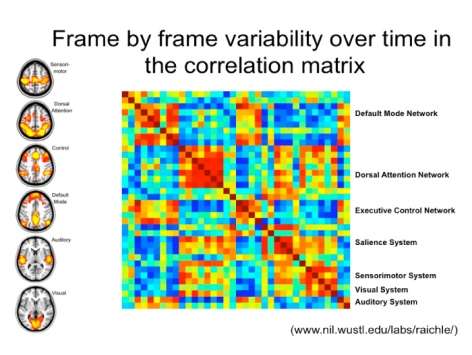
Download the Presentation (PDF, 2.2MB)Resting-state functional connectivity MRI is a relatively novel imaging approach that has gained popularity in the study of neuropsychiatric disorders. Functional connectivity in this setting is defined as sequential correlations in the spontaneous fMRI signal fluctuations between two or more regions. Using this approach, numerous groups have identified and begun to characterize a collection of brain networks referred to here as intrinsic connectivity networks (ICNs). The number of distinct, distinguishable ICNs has varied across different imaging laboratories, but is typically in the range of 10 to 20. This presentation focused on two ICNs in particular that Dr. Greicius's research group and others have linked to emotional salience processing and executive control. Linking ICNs with their accepted functions can be challenging, and several methods to do so were considered. The role of the salience network in disease states was also discussed.
Heritable Resting State Functional Connectivity: Toward Discovering the Foundations of Addiction Risk
F. Xavier Castellanos, M.D.
 Download the Presentation (PDF, 2.2MB)
Download the Presentation (PDF, 2.2MB)Neuroscientists are trying to determine the structural and functional connection patterns, or the "connectomes," of the human brain. These patterns can be studied at distinct scales, ranging from single synapses and minicolumns to anatomically distinct regions. The field has converged on studying anatomically distinct regions; however, there are still no widely accepted methods to divide the brain into relevant regions. Recently, investigators have noted that patterns of synchrony in spontaneous brain activity recapitulate the full range of functional circuits. Further, these patterns have sharp boundaries within any individual's brain. The resulting step functions allow division of the brain in terms of its built-in properties, but with unknown biological significance.
To determine their biological significance, Dr. Castellanos and his research team began by determining the likelihood that a trait is hereditary based on the differences in correlations between identical and fraternal twins. These tests included regional homogeneity, seed-based regression analyses, amplitude measures, and small-world network centrality. They found regionally specifiable relationships and characteristics that exhibit significant heritability, indicating a high likelihood that they were influenced by genetic factors. These results provide specific hypotheses for ongoing discovery of the genetic factors influencing brain structure and function. The ability to collect resting state scans across imaging centers and samples also promises to provide a framework for large-scale studies of the foundations of addiction risk, as repositories such as the International Neuroimaging Datasharing Initiative expand.
