If methamphetamine doesn't get into the brain, it can't cause psychoactive effects. That's the rationale for a novel treatment approach being pursued by NIDA-supported researchers at the University of Arkansas for Medical Sciences (UAMS) and the biotech company InterveXion Therapeutics (IXT).
Drs. Michael Owens and Brooks Gentry and colleagues have created a monoclonal antibody (mAb) that is intended to interact with methamphetamine and decrease its ability to enter the brain. They have successfully tested the antibody, called IXT-m200, in vitro and in animals and have now advanced its development to the second, penultimate stage of clinical trials. If all continues to go well, they hope to make it available to clinicians and patients in 3 to 5 years. This article describes their 20-year pathway to this point, and the work that remains.
An Anti-Methamphetamine Antibody
Antibodies in general help the immune system to detect and destroy foreign or harmful molecules. The UAMS-IXT team designed IXT-m200 using technology that enables researchers to fashion specific antibodies, the mAbs, against almost any molecule (see "How To Make a Monoclonal Antibody"). The specific type of mAbs they created—also known as chimeric antibodies—was built on mouse antibodies and engineered with pieces of human antibodies so that they pass muster with the human immune system and do not themselves induce an immune response. Plus, they maintain the ability to bind to methamphetamine tightly. When administered to a patient, the mAbs home in on their target molecule—in this case, methamphetamine—bind to it, and decrease its ability to rush into the brain.
Since their discovery in the 1970s, mAbs have served as research and diagnostic tools in the lab and in the clinic; today, some of them are used to treat cancer, rheumatoid arthritis, and numerous other diseases. Dr. Owens and his colleagues thought this binding strategy could also work to reduce the harmful effects of drugs of abuse. Once injected, IXT-m200 grabs and holds methamphetamine that is already in or subsequently enters the bloodstream. Because IXT-m200 is too large to pass through the blood-brain barrier, the captured drug stays in the blood, from which it can be delivered to the kidney and liver for elimination.
Dr. Owens explains how the researchers expect IXT-m200 to help patients overcome addiction, saying, "People use methamphetamine for the immediate, intense rush or high they get when they smoke, snort, or inject it. The antibody is expected to decrease those effects." The person's motivation to use the drug may then fall and their commitment to treatment and abstinence rise, especially when the patient is also in cognitive-behavioral ("talk") therapy.
The researchers foresee IXT-m200 being used primarily as an adjunct to help patients stay in behavioral therapy. Many patients with methamphetamine use disorder quit treatment prematurely, but studies have shown that the longer patients stay in treatment, the more likely they are to achieve sustained abstinence. Dr. Gentry says, "We envision a clinical scenario where we would give the antibody once a month in an outpatient setting. When a person relapses to methamphetamine use, the antibody would decrease the rate at which the drug rushes to the brain and so hopefully reduce its pleasurable reinforcing effects. We think the antibody will change the drug's effects so that patients will be more likely to return to rehab, shortening the process of giving up the drug."
Dr. Owens adds, "It's not a magic bullet, but it's an adjunct that nobody has been able to come up with yet."
The Beginning
Dr. Owens set out on the path to IXT-m200 in the 1980s. He had recently completed his Ph.D. at the University of North Carolina (UNC) and joined the laboratory of Dr. Michael Mayersohn at the University of Arizona. The Arizona group was studying how the body metabolizes the drug phencyclidine (PCP). Dr. Owens recalls, "Dr. Mayersohn said, 'Do you think you could develop antibodies to treat overdose?' I had just been to a lecture about monoclonal antibodies before I left UNC, and so I said, 'I think it might be possible, but you'd have to have monoclonal antibodies rather than simply polyclonals to achieve higher antibody concentrations and immediate effects.' And that started this whole process."
The Arizona team developed several mAbs against PCP, and Dr. Owens continued this work when he established his own laboratory at UAMS in 1986. While an excellent PCP antibody was discovered and successfully tested in animals, falling rates of PCP use and addiction caused them to abandon that project. The problem no longer warranted the extensive and expensive process of medication development.
In 1994, Dr. Gentry joined Dr. Owens' group. When methamphetamine use and addiction became rampant in the mid-1990s, the researchers saw a potential opportunity to put mAbs to good use. Says Dr. Gentry, "At the time, methamphetamine was very much a locally produced drug of abuse, and Arkansas was number one per capita in the United States in the number of meth labs. We recognized that addiction to this drug was a potentially long-term and long-acting problem, for which a long-acting antagonist, like a monoclonal antibody, could be effective."
In 1999, the researchers received their first grant from NIDA for this research and, 5 years later, they were confident enough that they were on the right track that they founded InterveXion to facilitate further drug development. Critical to the success of InterveXion has been the inclusion of three other established scientists, Drs. Ralph Henry, Misty Stevens, and Keith Ward. Together, this group realized that forming a company would allow them to apply for small business grants and also offer opportunities for larger grants that would be needed to conduct clinical trials. They also recognized that traditional pharmaceutical companies were unlikely to invest in mAb therapy for drug addiction without clinical proof-of-concept data. Their team was prepared to take the development into clinical trials to establish this proof of concept.
Learning From Animals
The team produced and evaluated about half a dozen of their best mAbs against methamphetamine. A key player in this process was Dr. Ivy Carroll of the Research Triangle Institute who synthesized all of the haptens for the antibody discovery process. To be an effective treatment, an antibody must bind the drug tightly (i.e., have a high affinity) and hold it in the bloodstream long enough for the body to break it down or eliminate it. Not all of the team's mAbs met these criteria equally well, especially when tested in laboratory animals rather than test tubes. "Early on, we found that our best antibody, which had the highest affinity we'd ever made, was inactivated once it was injected into the animals," Dr. Owens remembers.
After several years of creating and testing mAbs, Dr. Owens and colleagues determined that IXT-m200 had the most suitable attributes. Further in vitro and in vivo testing in rats established that IXT-m200 performed as desired without causing unwanted side effects. In animals, it:
- Had high affinity for methamphetamine and related stimulants (amphetamine, MDMA), but not for the body's own signaling molecules (neurotransmitters), important over-the-counter medications, or other drugs of abuse.
- Did not bind to molecules in the body that can trigger toxic reactions (i.e., complement-dependent cytotoxicity).
- Bound to methamphetamine in the blood, thereby preventing it from spreading to organs and particularly to the brain.
- Had a much longer half-life than methamphetamine in rats (about 9 days vs. 1 hour), indicating that it could bind to methamphetamine repeatedly; this suggested that the antibody would be effective even if a person took multiple methamphetamine doses to overcome the antibody's effects.
- Reduced the severity and duration of methamphetamine's stimulatory effects.
Dr. Owens and colleagues' in vitro and animal test results led to U.S. Food and Drug Administration approval to test IXT-m200 in people in 2013. The results of these clinical trials to date, and the future development process, are highlighted in part 2 of this article series.
How To Make a Monoclonal Antibody
The immune system uses antibodies to identify foreign or harmful molecules, bind to them, and thereby mark them for elimination from the body or for destruction by other components of the immune system. Normally, when the body encounters a foreign molecule, certain immune cells (B-cells) produce numerous slightly different antibodies that typically bind to different parts, or epitopes, of the foreign molecule.
In the 1970s, researchers learned how to isolate single B-cells that produced only one specific antibody recognizing one specific epitope. This allowed them to select and grow large numbers of cells that all produce the exact same antibody—the monoclonal antibodies (mAbs). Their main advantages are that they can be produced in large quantities and that they can be highly specific for a given target molecule, such as methamphetamine. In theory, monoclonal antibodies can be produced against almost any molecule of interest and lead to its detection, inactivation, or destruction, making them a valuable tool both for research and for treatment purposes.
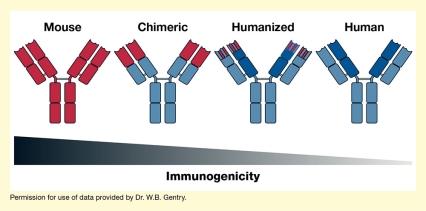
Scientists can also reengineer the mouse-produced antibodies to make them more human-like (i.e., replace mouse parts with human parts). This lowers the risk that the human immune system considers them as foreign and attacks them (i.e., reduces the antibodies' immunogenicity). Depending on the amount of human antibody parts introduced, the resulting mAbs are referred to as chimeric or humanized. InterveXion's IXT-m200 antibody is a chimeric mAb, which contains about two-thirds human material. As a result of these changes, the chimeric antibodies can be administered to human patients in whom they can capture methamphetamine but are much less immunogenic than mouse antibodies. mAb-based treatments are currently available for several disorders, including certain types of cancer and autoimmune disorders such as rheumatoid arthritis.
This research was supported by NIH grants DA031944, DA028915, DA037593, and DA045366.
- Text Description of Figure
The figure shows the general structure of four types of antibodies, including (from left to right) mouse, chimeric, humanized, and human antibodies. Antibody components derived from mouse are shown in red, and human-derived components are shown in blue. All antibodies have a Y-shaped structure, with four sections (arranged 2x2) making up the stem and four sections (arranged 2x2) each making up the two arms of the Y. For the antibody on the left, all 12 sections of the antibody are red, indicating that they are mouse derived. For the chimeric antibody (second from left), the four sections of the stem and the lower two sections of each arm are blue, indicating that they are human derived, and the two upper sections of each arm are red, indicating that they are mouse derived. For the humanized antibody (second from right), the four sections of the stem, the two lower sections of each arm, and half of the two upper sections of each arm are blue, indicating that there are human derived. The upper half of the upper two sections of each arm are striped red and blue, indicating that they are partially mouse derived and partially human derived. For the human antibody on the right, all sections are blue. A black-to-gray triangle pointing to the right underneath the four antibody molecules indicates progressively decreasing immunogenicity from mouse to chimeric, humanized, and human antibodies.
Source:
- Stevens, M.W., Tawney, R.L., West, C.M., et al. Preclinical characterization of an anti-methamphetamine monoclonal antibody for human use. mAbs 6(2):547-555, 2014.