As described in Part 1 of this series, Drs. Michael Owens and Brooks Gentry and colleagues at the University of Arkansas for Medical Sciences (UAMS) and the biotech company InterveXion Therapeutics (IXT) had created a monoclonal antibody (mAb) that is intended to help treat methamphetamine addiction by interacting with methamphetamine and decreasing its ability to enter the brain. They successfully tested the antibody, called IXT-m200, in vitro and in animals. The findings convinced the U.S. Food and Drug Administration (FDA) to approve testing of IXT-m200 in people in 2013.
Phase 1: IXT-m200 Is Well-Tolerated in People
To become an approved medication, IXT-m200 must, as all potential new medications usually do, pass three stages of human trials. A Phase 1 trial explores potential adverse effects of prospective new medications in humans for the first time. It also provides preliminary information about pharmacokinetics: how the medication is absorbed into the body, where it is distributed in the body, and how fast it is degraded and excreted.
For their Phase 1 study, the IXT team recruited 42 healthy volunteers who did not use methamphetamine. Each volunteer received a single injection of IXT-m200, with the individual doses successively increasing. The researchers then monitored the participants for more than 5 months, which is long enough to evaluate how long IXT-m200 remains in the body. The results were encouraging. No volunteer suffered any serious adverse reactions to the antibody. A few reported mild issues, only some of which seemed to be related to IXT-m200 administration, and all of which resolved on their own. As predicted from the animal studies and studies of other mAbs in humans, IXT-m200 stayed in the blood rather than dispersing into tissues. Its half-life was about 18 days, which meant that patients could potentially maintain therapeutic blood levels if dosed every 3 to 4 weeks.
Dr. Gentry explains, “The study showed that the antibody was indeed well-tolerated and, pharmacokinetically, the blood concentration profile over time was very much as we expected.” The FDA agreed, but raised an additional question: What if patients who were addicted to methamphetamine were to take more of the drug in an attempt to overcome IXT-m200’s effect? Could that cause any unexpected toxicity? The researchers chose to do additional studies to answer this question before proceeding to Phase 2 testing, which would be conducted with people who use methamphetamine.
Dr. Gentry says, “We needed to do some additional work with high doses of methamphetamine in animals to show that the antibody does not increase toxicity of methamphetamine.” Fortunately, these studies raised no new concerns, and the FDA gave the go-ahead for the next clinical trial.
Phase 2: Will IXT-m200 Redistribute Methamphetamine Into the Bloodstream?
Phase 2 clinical trials test whether a potential new medication produces the proposed therapeutic pharmacological effects in the human body that the developers have seen in animal models. They can also add to the evidence for safety that was gathered in the Phase 1 trial.
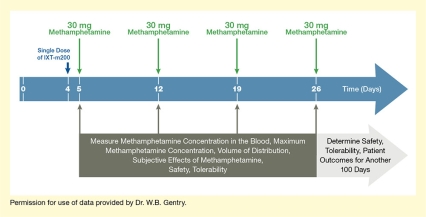
In their first Phase 2 trial, which the team dubbed STAMPOUT (Study of Antibody for Methamphetamine Outpatient Therapy), Dr. Gentry and colleagues are seeking to establish how efficiently IXT-m200 captures methamphetamine in the blood and prevents it from causing its pleasurable effects. The trial participants are people who use methamphetamine but are not seeking treatment. Each participant receives weekly doses of methamphetamine for up to 4 weeks. Between the first and second doses, the patient receives either IXT-m200 or placebo (see Figure 1). After each dose, the researchers take serial blood samples to determine how much of the methamphetamine is detectable in the blood and for how long. They also ask the participants about how they perceive the drug’s effects and monitor for adverse reactions for another 3 months.
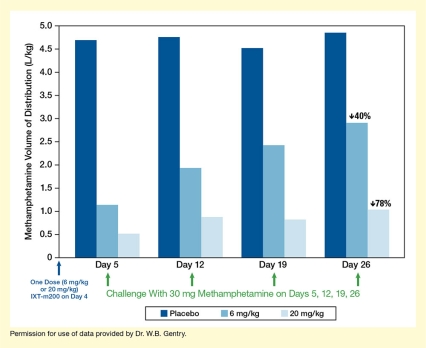
The IXT team recently released preliminary trial results, using data from the first 36 patients to complete the protocol. Their analyses indicate that among those patients, IXT-m200 efficiently trapped and held methamphetamine in the blood (see Figure 2). The maximum concentrations and the cumulative amount of drug in the blood over time were both greater with IXT-m200 than before treatment or with placebo. Dr. Gentry is very excited about these findings. “These interim data are the first evidence that IXT-m200 alters methamphetamine pharmacokinetics in actual methamphetamine users,” he says.
Moreover, the data suggest that IXT-m200 survives in the blood long enough for patients to need only monthly doses. That’s valuable, Dr. Gentry explains, because “if patients miss a dose by a few days, it doesn’t matter as much. Monthly dosing requires less day-to-day decision-making on the part of the patient.”
Buoyed by their preliminary results, Dr. Owens and colleagues are continuing the STAMPOUT study and anticipate completing it within a year. If the results with the full study cohort bear out the promise of the preliminary results, and no new problems surface, they will confer with the FDA to design additional studies.
Next Phase: Will IXT-m200 Help Patients Recover?
If Phase 1 and 2 trials show that a potential new medication is well-tolerated and causes the desired pharmacological effects, the question still remains: Will it help patients? That’s the question later trials ask. For IXT-m200, it translates to: Will it enhance abstinence and improve quality of life for people addicted to methamphetamine?
If IXT-m200 advances to later trials, it will be the first time that actual patients who seek to become abstinent receive the mAb. As of today, the researchers are projecting a basic study design in which they will randomly assign people who use methamphetamine and are seeking treatment to receive either placebo or IXT-m200 monthly for 6 months. Dr. Owens estimates that even if the team completes STAMPOUT in 1 year, finishing later-stage trials will take until at least 2022 or 2023.
Dr. Gentry adds, “Our best estimate right now is that we’re about 5 years away from bringing IXT-m200 to market. There’s a lot that has to go into completing all the steps that the FDA requires for a product approval. We’re looking for ways to accelerate the program and other potential support mechanisms to move it along as quickly as we can.”
The FDA has signaled that it will do what it can to speed the process along. The agency has recognized methamphetamine addiction as a serious condition with unmet medical need and accordingly granted IXT-m200 “Fast-Track” priority in its development status.
A Marathon, Not a Sprint
IXT-m200 is the first proposed medication for methamphetamine addiction ever to advance in the development process as far as a Phase 2 trial. (To read about another NIDA-supported medication development project for methamphetamine addiction, see our Narrative of Discovery series, Parts 1 to 4). Still, Dr. Owens and colleagues had hoped to be further along by now. “I thought it would be a lot faster,” admits Dr. Owens. “I think several of us are a bit surprised at the length of time needed to get through the clinical development process.”
The researchers say that NIDA’s continuing support has been vital. They are grateful to have received infrastructural and other nonmonetary support from UAMS over the years. However, Dr. Owens says, “The only dollars we’ve ever had into the company have been from NIDA.”
Dr. Gentry agrees. “NIDA has been very supportive and provided funding mechanisms that have allowed us to progress from basic research to clinical trials. This level of support has been very beneficial to us and others in the field who are trying to treat and cure substance use disorders,” he notes.
While it has taken many years to get to this point in the medication development process, the researchers say they are happy with the path they’ve taken. Says Dr. Gentry, “I wanted to take on a project that that, if successful, would have an impact on human health, and we’ve done that.”
Dr. Owens adds, “We’re nothing if not optimistic. We wouldn’t have done this for as long as we have if we weren’t optimistic that it was going to work.”
This research was supported by NIH grants DA031944, DA028915, DA037593, and DA045366.
- Text Description of Figure 1
The figure illustrates the basic design of the Phase 2 STAMPOUT trial of the IXT-m200 mAb against methamphetamine. The blue horizontal arrow indicates time in days, with Days 0, 4, 5, 12, 19, and 26 marked by white vertical lines. A dark blue arrow pointing down at Day 4 indicates administration on this day of a single dose of IXT-m200 or placebo. Four green arrows pointing down at Days 5, 12, 19, and 26 indicate administration on these days of 30 mg methamphetamine. Dark gray arrows pointing up from a dark gray box at Days 5, 12, 19, and 26 indicate analyses on these days of methamphetamine concentration in the blood, maximum methamphetamine concentration, volume of distribution, subjective effects of methamphetamine, safety, and tolerability. A light gray arrow pointing to the right underneath the blue arrow indicates that the safety and tolerability of IXT-m200 and patient outcomes were determined for another 100 days.
- Text Description of Figure 2
The bar chart shows the distribution of methamphetamine in the body resulting from methamphetamine challenge on different days after treatment with placebo or two different doses of IXT-m200 mAb. The horizontal x-axis represents different time points. A single blue arrow underneath the y-axis indicates administration of a single dose of 6 mg/kg or 20 mg/kg IXT-m200 or placebo on Day 0. The four groups of three bars each show results for Days 5, 12, 19, and 26, respectively (from left to right). Dark blue bars indicate results after administration of placebo, medium blue bars indicate results after administration of 6 mg/kg IXT-m200, and light blue bars indicate results after administration of 20 mg/kg IXT-m200 on Day 0. A green arrow underneath each group of bars indicates a challenge with 30 mg methamphetamine on those days. The vertical y-axis shows the methamphetamine volume of distribution in L/kg on a scale from 0 to 5.0.
On Day 5, the volume of distribution of methamphetamine was about 4.6 L/kg for participants receiving placebo, about 1.1 L/kg for those receiving 6 mg/kg IXT-m200, and about 0.5 L/kg for those receiving 20 mg/kg IXT-m200. On Day 12, the volume of distribution was about 4.7 L/kg for participants receiving placebo, about 1.9 L/kg for those receiving 6 mg/kg IXT-m200, and about 0.8 L/kg for those receiving 20 mg/kg IXT-m200. On Day 19, the volume of distribution was about 4.5 L/kg for participants receiving placebo, about 2.5 L/kg for those receiving 6 mg/kg IXT-m200, and about 0.8 L/kg for those receiving 20 mg/kg IXT-m200. On Day 26, the volume of distribution was about 4.8 L/kg for participants receiving placebo, about 2.9 L/kg for those receiving 6 mg/kg IXT-m200, and about 1.0 L/kg for those receiving 20 mg/kg IXT-m200.
Source:
- Stevens, M.W., Tawney, R.L., West, C.M., et al. Preclinical characterization of an anti-methamphetamine monoclonal antibody for human use. mAbs 6(2):547-555, 2014.