"We’re so close. We’ve come such a long way. It’s so close I can taste it."
Dr. Linda Dwoskin feels just one small step away from success in her effort to develop the first-ever medication to treat methamphetamine addiction. She and colleagues at the Universities of Kentucky and Arkansas have created a molecule that blocks methamphetamine’s addictive effects and completed preclinical testing without raising any red flags for undesirable side effects.
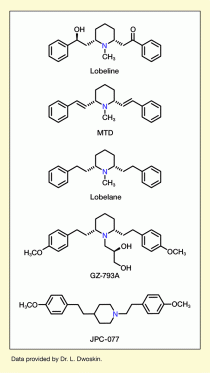
Dr. Dwoskin has been working toward this goal since the mid-1990s. Part 1 of this Narrative of Discovery related her initial insight that a molecule called lobeline, found in the Lobelia plant, could help people overcome methamphetamine addiction. Part 2 related how, when lobeline proved nonviable as a medication, she and colleagues at the Universities of Kentucky and Arkansas initiated a program to modify and improve the molecule.
This installment describes the team’s experiences with four lobeline variants. Each one advanced further than the previous one through the extensive course of in vitro, animal-tissue, and whole-animal assays that a potential medication must pass to qualify for testing in people. Finally, in 2017, the fourth variant crossed the finish line.
Intolerable Tolerance
The researchers’ first significant advance after lobeline failed was meso-transdiene (MTD). When tested in vitro, MTD, like lobeline, blocked methamphetamine-induced dopamine release, the mechanism that underlies the drug’s rewarding effect. However, further testing indicated that MTD could have abuse and addiction liability of its own. This finding eliminated MTD from further consideration.
The researchers’ next highly promising molecule, called lobelane, equaled or surpassed MTD’s results on all in vitro tests and avoided the pharmacological effect that made MTD potentially abusable. Dr. Dwoskin’s team moved lobelane into live animal testing.
Dr. Michael Bardo, a behavioral pharmacologist at the University of Kentucky, directs animal testing for the project. The first two tests he and his team performed confirmed that lobelane’s favorable results in vitro translated into desirable changes in animal behavior. In the tests:
- Pretreating rats with lobelane reduced methamphetamine-stimulated locomotor activity, which is considered a stand-in for a drug’s rewarding effect in people.
- Rats that were allowed to freely self-administer methamphetamine took less of the drug after being treated with lobelane.
The next behavioral test addressed whether animals would develop tolerance to lobelane. Medication tolerance is a common phenomenon. A medication works well the first time someone takes it, but its effectiveness wanes with repeated exposures. This is not a problem for medications that people take for a limited time for an acute condition. It would be disastrous, however, for a medication that must be taken long-term to treat a chronic condition such as addiction.

Dr. Dwoskin estimates that a medication to treat methamphetamine addiction will need to protect patients from the drug’s rewarding effects for a year or longer. Patients’ risk of re-exposing themselves to the drug is high throughout the first year of abstinence, when their craving can be severe and they may still be learning skills for drug-free living. If the medication loses efficacy during that time, such lapses are highly likely to precipitate full relapses.
To test for tolerance, Dr. Bardo’s team gave rats access to methamphetamine in 2-hour daily sessions. Once the rats established a steady pattern of self-administration of the drug, the researchers treated them with lobeline while continuing to give them access to the drug in 7 additional daily sessions. The result knocked lobelane out of the running. The rats initially reduced their methamphetamine intake, but within 4 to 5 days reverted toward pre-treatment levels.
Dr. Dwoskin explains, "The body typically tries to go back to equilibrium, so it tries to counteract whatever a drug is doing to it." She speculates that the body probably responds to repeated lobelane exposure by stepping up production of enzymes that metabolize the molecule. As these enzymes build up, they break down increasing amounts of lobelane in the bloodstream before it reaches the brain tissues that are the site of its potentially therapeutic activity. Alternatively, Dr. Dwoskin says, the brain might produce larger amounts of lobelane’s target protein (vesicular monoamine transporter 2 [VMAT2]).
Heart Stopper
The team went back to work. Lobelane had survived through all the researchers’ in vitro tests, and halfway through their roster of animal tests. One year later, a new molecule sailed through every test save the very last.
The team designated the compound GZ-793A, combining the initials of postdoctoral fellow Dr. Guangrong Zheng, who performed early work on it, and the page in his lab daybook where he recorded its structure. Dr. Dwoskin says, "GZ-793A was stellar in all the in vitro and animal assays. It blocked the primary reinforcing effect of methamphetamine and cue-induced reinstatement of drug-taking. It did not produce rewarding effects of its own. Animals did not develop tolerance to it. It was effective when given orally."
The researchers were elated. After more than 15 years of trying, Dr. Dwoskin and her team had developed a molecule that passed every relevant test that they were equipped to perform in their lab.
"We were thrilled, ready to pop the corks on the champagne. We thought we had the drug," Dr. Dwoskin says. The team began assembling an application for funds to enable them to test GZ-793A in people. Meanwhile, they sent samples of GZ-793A to NIDA, which forwarded them to an outside laboratory for one additional test.
The test result kept the bubbly in the cooler. The U.S. Food and Drug Administration (FDA) would never approve GZ-793A for human use.
The test showed that GZ-793A changed ion flow through a cardiac protein pore called the hERG channel. In the late 1990s, investigators identified pharmacological alteration of hERG activity as the cause of a sometimes-fatal cardiac arrhythmia, called torsade des pointes, that was occurring among people using a popular diet drug called fenfluramine. The FDA ordered fenfluramine withdrawn and has since declined to approve any new drug with hERG activity.
"NIDA was really great," Dr. Dwoskin says. "They gave GZ-793A every chance. They tested for the hERG effect in three different assays, because a single assay can give you a misleading answer." In this case, however, the results were consistent. GZ-793A produced an hERG effect in human embryonic kidney cells, rabbit cardiac cells, and live rabbits. Moreover, it did so in the same concentrations that produced GZ793A’s potentially therapeutic effect.
"Bummer of bummers," says Dr. Dwoskin. "That was just incredibly disheartening. Talk about a depressed person there for a while."
Dr. Dwoskin pauses, then continues, "Well, that’s happened to us over and over, with different problems that we’ve had to overcome. You just have to go back to the drawing board and figure out a series of steps that seem reasonable to get rid of the problem."
Almost Home?
Dr. Dwoskin applied for and received a new grant from NIDA. It was not, as she had hoped, to advance GZ-793A into human trials. Instead, it was to find a way around the hERG problem.
In contrast to most of the problems Dr. Dwoskin and her team had encountered, there was already a body of research on how to fix this one. Dr. Dwoskin recounts, "When hERG was first identified, big pharmaceutical companies realized that many of the compounds they were developing had hERG activity. So, they researched ways to get around it. By the time we encountered the problem, they had found four or five changes that you could make to a molecule, either singly or in combination, that worked." Following these leads, the team quickly came up with a molecule that passed all tests, maintaining all of GZ-793A’s valuable properties and showing no hERG activity.
The molecule, called JPC-077 (using the initials of Ph.D. student John Paul Culver) must overcome one last hurdle to reach the preclinical development finish line and enter human trials. Dr. Dwoskin does not believe that the hurdle will prove to be very high.
She explains, "The issue is that to be effective, JPC-077 needs to be given in a dose that is too large for oral administration. There are lots of precedents for fixing this type of problem, so I am confident that we can succeed."
Recalling all the cycles of mounting hope and disappointment she has experienced to date, Dr. Dwoskin says, "It takes a certain kind of person to be able to stick to the drug development pathway all the way through. There’s a lot of banging your head against the wall because things don’t always work the way you want them to. [In this Narrative of Discovery,] we’ve only been talking about our highlights, the lead compounds that looked like they had lots of promise. Between any two of these, the people in my lab ran through a whole bunch of compounds that didn’t look good. You have to be able to tolerate not getting good ones a lot of the time."
But now, she’s convinced, "We’re so close."
Read Part 4 of this Narrative of Discovery.
Resource Conservation
With every test a molecule passes, it confirms its therapeutic potential or checks off another prerequisite for clinical testing in people. Researchers’ hopes for success this time rise.
At the same time, researchers know that an overwhelming majority of compounds that enter preclinical testing will fail. And, the further a molecule goes in testing before it fails, the more resources have been expended on a dead end.
For this reason, Dr. Dwoskin’s team test their candidate molecules in a sequence that is designed to frontload less costly assays and spot fatal flaws as soon and economically as possible. Following this principle, after the late failure of GZ793A, they imported hERG testing into their lab, and now include it in their early in vitro testing.
The team’s testing sequence aims to learn as much as possible through in vitro testing before proceeding to testing in animals. Dr. Dwoskin says, "Animal tests are more technically demanding, time consuming, and resource intensive. For example, for reliable results, you need about 8 animals to complete each animal test, but you need to start out with more, because some drop out along the way. Things happen—intravenous lines get clogged, or you have a problem with the implant [that secures the intravenous line to the animal], and so on."
Dr. Dwoskin estimates that Dr. Bardo used about two dozen rats and spent a year testing lobelane in various doses before the tolerance problem emerged that killed hopes for the molecule. Since that experience, she says, "We really would like to figure out an in vitro test that will tell us whether animals will develop tolerance to a molecule."
- Text Description of Figure
-
The figure shows the molecular structures of lobeline (top) and its successors MTD (second from the top), lobelane (middle), GZ-793A (second from bottom), and JPC-077 (bottom). The molecules have a similar basic structure but were modified by the addition or removal of different chemical groups to modulate their effectiveness and risk of side effects.