Organizational Chart

- Description of Organizational Chart
-
Office of the Director
Nora D. Volkow, M.D. - Director
Wilson Compton, M.D., M.P.E. - Deputy Director
Glenda Conroy, MBA, CPA, PMP - Associate Director for ManagementOffice of Management
Glenda Conroy, MBA, CPA, PMP - Director
Office of Extramural Affairs
Mark Swieter, Ph.D. - Acting Director
Office of Science Policy and Communications
Jack B. Stein, Ph.D. - Director
Division of Clinical Neuroscience and Behavioral Research
Joseph Frascella, Ph.D. - Director
Division of Epidemiology, Services and Prevention Research
Redonna Chandler, Ph.D. - Acting Director
Intramural Research Program
Antonello Bonci, M.D. - Scientific Director
Center for the Clinical Trials Network
Betty Tai, Ph.D. - Director
Division of Basic Neurosciences and Behavioral Research
Joni Rutter, Ph.D. - Director
Division of Pharmacotherapies and Medical Consequences of Drug Abuse
Phil Skolnick, Ph.D., D.Sc. - Director
Appropriation Language
For carrying out section 301 and title IV of the PHS Act with respect to drug abuse, [$1,025,435,000] $1,023,268,000.
Tables
- Amounts Available for Obligation Table
-
Amounts Available for Obligation 1
(Dollars in Thousands)Source of Funding FY 2013
ActualFY 2014
EnactedFY 2015
President's
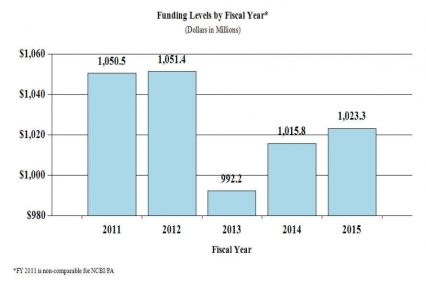
BudgetAppropriation $1,053,367 $1,025,435 $1,023,268 Type 1 Diabetes 0 0 0 Rescission -2,107 0 0 Sequestration -52,872 0 0 Subtotal, adjusted appropriation $998,389 $1,025,435 $1,023,268 FY 2013 Secretary's Transfer -5,824 0 0 OAR HIV/AIDS Transfers 0 -8,270 0 Comparative transfers to NLM for NCBI and Public Access -1,179 -1,411 0 National Children's Study Transfers 847 0 0 Subtotal, adjusted budget authority $992,233 $1,015,754 $1,023,268 Unobligated balance, start of year 0 0 0 Unobligated balance, end of year 0 0 0 Subtotal, adjusted budget authority $992,233 $1,015,754 $1,023,268 Unobligated balance lapsing -8 0 0 Total obligations $992,225 $1,015,754 $1,023,268 1 Excludes the following amounts for reimbursable activities carried out by this account:
FY 2013 - $81,601 FY 2014 - $52,659 FY 2015 - $57,706
- Budget Mechanism Table
-
Budget Mechanism - Total1 (Dollars in Thousands) Mechanism FY 2013
ActualFY 2014
Enacted2FY 2015
President's
BudgetFY 2015
+/-
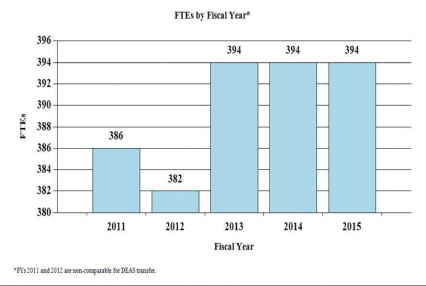
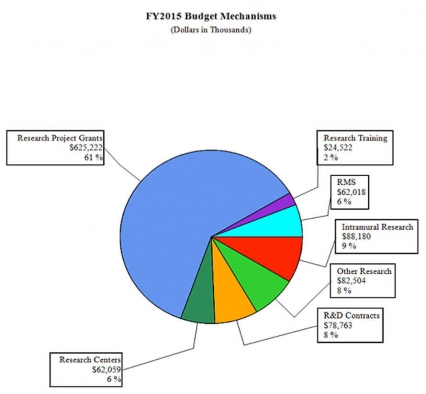
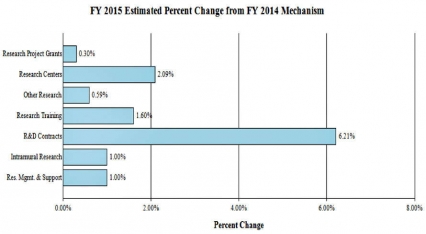
FY 2014No. Amt. No. Amt. No. Amt. No. Amt. Research Projects: Noncompeting 985 $432,156 986 $464,521 880 $418,784 -106 -$45,737 Administrative Supplements (134) 12,802 (84) 5,000 (84) 5,000 (0) 0 Competing: Renewal 40 19,581 37 18,127 34 16,658 -3 -1,469 New 324 123,187 292 113,578 413 161,567 121 47,989 Supplements 1 122 2 250 2 250 0 0 Subtotal, competing 365 $142,890 331 $131,955 449 $178,475 118 $46,520 Subtotal, RPGs 1,350 $587,847 1,317 $601,476 1,329 $602,259 12 $783 SBIR/STTR 51 20,009 54 21,890 59 22,963 5 1,073 Research Project Grants 1,401 $607,856 1,371 $623,366 1,388 $625,222 17 $1,856 Research Centers: Specialized / Comprehensive 37 $60,789 33 $60,789 33 $62,059 0 $1,270 Clinical Research 0 0 0 0 0 0 0 0 Biotechnology 0 0 0 0 0 0 0 0 Comparative Medicine 0 0 0 0 0 0 0 0 Research Centers in Minority Institutions 0 0 0 0 0 0 0 0 Research Centers 37 $60,789 33 $60,789 33 $62,059 0 $1,270 Other Research: Research Careers 201 $33,698 205 $34,372 207 $34,716 2 $344 Cancer Education 0 0 0 0 0 0 0 0 Cooperative Clinical Research 13 32,211 13 32,211 13 32,211 0 0 Biomedical Research Support 0 0 0 0 0 0 0 0 Minority Biomedical Research Support 0 1,311 0 1,311 0 1,311 0 0 Other 64 13,162 69 14,125 69 14,266 0 141 Other Research 278 $80,383 287 $82,019 289 $82,504 2 $485 Total Research Grants 1,716 $749,027 1,691 $766,174 1,710 $769,785 19 $3,611 Ruth L. Kirschstein Training Awards FTTPs FTTPs FTTPs FTTPs Individual Awards 153 $5,947 151 $5,947 151 $6,043 0 $96 Institutional Awards 377 18,188 371 18,188 371 18,479 0 291 Total Research Training 530 $24,136 522 $24,135 522 $24,522 0 $387 Research and Development Contracts 74 $74,391 74 $74,160 74 $78,763 0 $4,603 SBIR/STTR (non-add) (15) (5,848) (15) (5,848) (15) (5,848) (0) (0) Intramural Research 125 84,768 125 87,307 125 88,180 0 873 Research Management and Support 269 59,910 269 61,404 269 62,018 0 614 Research Management and Support (SBIR Admin) (non-add) (0) (32) (0) (40) (0) (40) (0) (0) Construction 0 0 0 0 Buildings and Facilities 0 0 0 0 Total, NIDA 394 $992,232 394 $1,015,754 394 $1,023,268 0 $7,514 1 All items in italics and brackets are non-add entries. FY 2013 and FY 2014 levels are shown on a comparable basis to FY 2015.
2 The amounts in the FY 2014 column take into account funding reallocations, and therefore may not add to the total budget authority reflected herein.
- Summary of Changes Table
-
Summary of Changes1
(Dollars in Thousands)FY 2014 Enacted $1,015,754 FY 2015 President's Budget $1,023,268 Net change $7,514 CHANGES 2015 President's Budget: FTEs 2015 President's Budget: Budget Authority Change from FY 2014: FTEs Change from FY 2014: Budget Authority A. Built-in: 1. Intramural Research: a. Annualization of January 2014 pay increase & benefits $24,326 $89 b. January FY 2015 pay increase & benefits 24,326 269 c. Zero more days of pay (n/a for 2015) 24,326 0 d. Differences attributable to change in FTE 24,326 0 e. Payment for centrally furnished services 11,036 184 f. Increased cost of laboratory supplies, materials, other expenses, and non-recurring costs 52,817 631 Subtotal $1,173 2. Research Management and Support: a. Annualization of January 2014 pay increase & benefits $37,182 $139 b. January FY 2015 pay increase & benefits 37,182 420 c. Zero more days of pay (n/a for 2015) 37,182 0 d. Differences attributable to change in FTE 37,182 0 e. Payment for centrally furnished services 3,024 51 f. Increased cost of laboratory supplies, materials, other expenses, and non-recurring costs 21,811 748 Subtotal $1,358 Subtotal, Built-in $2,531 Summary of Changes - Continued1
(Dollars in Thousands)CHANGES Fy 2015 President's Budget: No. Fy 2015 President's
Budget: AmountChange from
FY 2014: No.Change from
FY 2014: AmountB. Program: 1. Research Project Grants: a. Noncompeting 880 $423,784 -106 -$45,737 b. Competing 449 178,475 118 46,520 c. SBIR/STTR 59 22,963 5 1,073 Subtotal, RPGs 1,388 $625,222 17 $1,856 2. Research Centers 33 $62,059 0 $1,270 3. Other Research 289 82,504 2 485 4. Research Training 522 24,522 0 387 5. Research and development contracts 74 78,763 0 4,603 Subtotal, Extramural $873,070 $8,601 FTEs FTEs 6. Intramural Research 125 $88,180 0 -$300 7. Research Management and Support 269 62,018 0 -744 8. Construction 0 0 9. Buildings and Facilities 0 0 Subtotal, program 394 $1,023,268 0 $7,557 Total changes $7,514 1 The amounts in the Change from FY 2014 column take into account funding reallocations, and therefore may not add to the net change reflected herein.
-
Budget Authority by Activity1
(Dollars in thousands)Extramural Research FY 2013
ActualFY 2014
Enacted2FY 2015
President's
BudgetFY 2015
+/-
FY 2014FTE Amount FTE Amount FTE Amount FTE Amount Detail: Basic and Clinical Neuroscience and Behavioral Research $440,895 $435,547 $440,720 $5,173 Epidemiology, Services and Prevention Research 241,661 252,017 253,817 1,800 Pharmacotherapies and Medical Consequences 120,490 131,897 133,025 1,128 Clinical Trials Network 44,508 45,008 45,508 500 Subtotal, Extramural $847,554 $864,469 $873,070 $8,601 Intramural Research 125 $84,768 125 $87,307 125 $88,180 0 $873 Research Management & Support 269 $59,910 269 $61,404 269 $62,018 0 $614 TOTAL 394 $992,232 394 $1,015,754 394 $1,023,268 0 $7,514 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
2The amounts in the FY 2014 column take into account funding reallocations, and therefore may not add to the total budget authority reflected herein.
-
Authorizing Legislation PHS Act/Other Citation U.S. Code Citation 2014 Amount Authorized FY 2014 Enacted 2015 Amount Authorized FY 2015 President's Budget Research and Investigation Section 301 42§241 Indefinite $1,015,754,000 Indefinite $1,023,268,000 National Institute on Drug Abuse Section 401(a) 42§281 Indefinite Indefinite Total, Budget Authority $1,015,754,000 $1,023,268,000
- Appropriations History Table
-
Appropriations History Fiscal Year Budget Estimate to Congress House Allowance Senate Allowance Appropriation 2005 $1,019,060,000 $1,019,060,000 $1,026,200,000 $1,014,760,000 Rescission ($8,341,000) 2006 $1,010,130,000 $1,010,130,000 $1,035,167,000 $1,010,130,000 Rescission ($10,101,000) 2007 $994,829,000 $994,829,000 $1,000,342,000 $1,000,621,000 Rescission $0 2008 $1,000,365,000 $1,015,559,000 $1,022,594,000 $1,018,493,000 Rescission ($17,793,000) Supplemental $5,322,000 2009 $1,001,672,000 $1,035,997,000 $1,029,539,000 $1,032,759,000 Rescission $0 2010 $1,045,384,000 $1,069,583,000 $1,050,091,000 $1,059,848,000 Rescission $0 2011 $1,094,078,000 $1,092,369,000 $1,059,848,000 Rescission ($9,306,097) 2012 $1,080,018,000 $1,080,018,000 $1,038,714,000 $1,055,362,000 Rescission ($1,994,634) 2013 $1,054,001,000 1,057,196,000 $1,053,367,366 Rescission ($2,106,735) Sequestration ($52,871,798) 2014 $1,071,612,000 $1,064,490,000 $1,025,435,000 Rescission $0 2015 $1,023,268,000
-
Budget Authority by Object Class1
(Dollars in Thousands)FY 2014 Enacted FY 2015 President's Budget Fy 2015 +/- Fy 2014 Total compensable workyears: Full-time employment 394 394 0 Full-time equivalent of overtime and holiday hours 103 103 0 Average ES salary $180 $181 $2 Average GM/GS grade 12.5 12.5 0.0 Average GM/GS salary $108 $109 $1 Average salary, grade established by act of July 1, 1944 (42 U.S.C. 207) $108 $109 $1 Average salary of ungraded positions $111 $112 $1 OBJECT CLASSES FY 2014 Enacted FY 2015 President's Budget Fy 2015 +/- Fy 2014 Personnel Compensation: 11.1 Full-time permanent $30,164 $30,466 $302 11.3 Other than full-time permanent 10,959 11,068 110 11.5 Other personnel compensation 813 821 8 11.7 Military personnel 932 942 9 11.8 Special personnel services payments 4,700 4,747 47 11.9 Subtotal Personnel Compensation $47,567 $48,043 $476 12.1 Civilian personnel benefits $12,455 $12,891 $436 12.2 Military personnel benefits 567 573 6 13.0 Benefits to former personnel 0 0 0 Subtotal Pay Costs $60,590 $61,507 $917 21.0 Travel and transportation of persons $832 $832 $0 22.0 Transportation of things 186 186 0 23.1 Rental payments to GSA 0 0 0 23.2 Rental payments to others 0 0 0 23.3 Communications, utilities and miscellaneous charges 497 497 0 24.0 Printing and reproduction 5 5 0 25.1 Consulting services $1,687 $1,687 $0 25.2 Other services 4,300 4,300 0 25.3 Purchase of goods and services from government accounts 116,487 122,044 5,557 25.4 Operation and maintenance of facilities $1,162 $312 -$850 25.5 Research and development contracts 35,731 35,088 -644 25.6 Medical care 1,299 1,299 0 25.7 Operation and maintenance of equipment 1,371 1,371 0 25.8 Subsistence and support of persons 0 0 0 25.0 Subtotal Other Contractual Services $162,036 $166,100 $4,063 26.0 Supplies and materials $4,011 $3,813 -$198 31.0 Equipment 3,639 3,643 4 32.0 Land and structures 0 0 0 33.0 Investments and loans 0 0 0 41.0 Grants, subsidies and contributions 783,958 786,685 2,727 42.0 Insurance claims and indemnities 0 0 0 43.0 Interest and dividends 0 0 0 44.0 Refunds 0 0 0 Subtotal Non-Pay Costs $955,164 $961,761 $6,597 Total Budget Authority by Object Class $1,015,754 $1,023,268 $7,514 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
- Salaries and Expenses Table
-
Salaries and Expenses
(Dollars in Thousands)OBJECT CLASSES FY 2014 Enacted FY 2015 President's Budget FY 2015 +/- FY 2014 Personnel Compensation: Full-time permanent (11.1) $30,164 $30,466 $302 Other than full-time permanent (11.3) 10,959 11,068 110 Other personnel compensation (11.5) 813 821 8 Military personnel (11.7) 932 942 9 Special personnel services payments (11.8) 4,700 4,747 47 Subtotal Personnel Compensation (11.9) $47,567 $48,043 $476 Civilian personnel benefits (12.1) $12,455 $12,891 $436 Military personnel benefits (12.2) 567 573 6 Benefits to former personnel (13.0) 0 0 0 Subtotal Pay Costs $60,590 $61,507 $917 Travel and transportation of persons (21.0) $832 $832 $0 Transportation of things (22.0) 186 186 0 Rental payments to others (23.2) 0 0 0 Communications, utilities and miscellaneous charges (23.3) 497 497 0 Printing and reproduction (24.0) 5 5 0 Other Contractual Services: Consultant services (25.1) 1,687 1,687 0 Other services (25.2) 4,300 4,300 0 Purchases from government accounts (25.3) 74,102 74,601 499 Operation and maintenance of facilities (25.4) 1,162 312 -850 Operation and maintenance of equipment (25.7) 1,371 1,371 0 Subsistence and support of persons (25.8) 0 0 0 Subtotal Other Contractual Services $82,622 $82,271 -$351 Supplies and materials (26.0) $4,011 $3,813 -$198 Subtotal Non-Pay Costs $88,152 $87,604 -$549 Total Administrative Costs $148,742 $149,111 $368
- Detail of Full-Time Equivalent Employment (FTEs) Table
-
Details of Full-Time Equivalent Employment (FTEs) OFFICE/DIVISION FY 2013 Actual FY 2014 Est. FY 2015 Est. Civilian Military Total Civilian Military Total Civilian Military Total Center for the Clinical Trials Network Direct: 15 1 16 15 1 16 15 1 16 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 15 1 16 15 1 16 15 1 16 Division of Basic Neuroscience & Behavioral Research Direct: 29 0 29 29 0 29 29 0 29 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 29 0 29 29 0 29 29 0 29 Division of Clinical Neuroscience and Behavioral Research Direct: 15 0 15 15 0 15 15 0 15 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 15 0 15 15 0 15 15 0 15 Division of Epidemiology, Services and Prevention Research Direct: 29 2 31 29 2 31 29 2 31 Reimbursable: 1 0 1 1 0 1 1 0 1 Total: 30 2 32 30 2 32 30 2 32 Division of Pharmacotherapies and Medical Consequences of Drug Abuse Direct: 30 0 30 30 0 30 30 0 30 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 30 0 30 30 0 30 30 0 30 Intramural Research Program Direct: 121 3 124 121 3 124 121 3 124 Reimbursable: 1 0 1 1 0 1 1 0 1 Total: 122 3 125 122 3 125 122 3 125 Office of Extramural Affairs Direct: 18 0 18 18 0 18 18 0 18 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 18 0 18 18 0 18 18 0 18 Office of Management Direct: 57 0 57 57 0 57 57 0 57 Reimbursable: 24 0 24 24 0 24 24 0 24 Total: 81 0 81 81 0 81 81 0 81 Office of Science Policy and Communication Direct: 27 0 27 27 0 27 27 0 27 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 27 0 27 27 0 27 27 0 27 Office of the Director Direct: 21 0 21 21 0 21 21 0 21 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 21 0 21 21 0 21 21 0 21 Total (Includes FTEs whose payroll obligations are supported by the NIH Common Fund) 388 6 394 388 6 394 388 6 394 FTEs supported by funds from Cooperative Research and Development Agreements 0 0 0 0 0 0 0 0 0 Fiscal Year Average GS Grade 2011 13.0 2012 12.8 2013 12.4 2014 12.5 2015 12.5
- Detail of Positions Table
-
Detail of Positions GRADE FY 2013 Actual FY 2014 Enacted FY 2015 President's Budget Total, ES Positions 1 1 1 Total, ES Salary 177,884 179,663 181,460 GM/GS-15 152,276 153,799 155,337 GM/GS-14 129,879 131,178 132,490 GM/GS-13 106,071 107,132 108,203 GS-12 89,222 90,114 91,015 GS-11 69,881 70,580 71,286 GS-10 73,917 74,656 75,403 GS-9 59,711 60,308 60,911 GS-8 56,871 57,440 58,014 GS-7 47,750 48,228 48,710 GS-6 49,375 49,869 50,367 GS-5 39,184 39,576 39,972 GS-4 28,375 28,659 28,945 GS-3 27,130 27,401 27,675 GS-2 24,865 25,114 25,365 GS-1 0 0 0 Subtotal 954,507 964,054 973,693 Grades established by Act of July 1, 1944 (42 U.S.C. 207): 0 0 0 Assistant Surgeon General 0 0 0 Director Grade 116,536 117,701 118,878 Senior Grade 89,100 89,991 90,891 Full Grade 0 0 0 Senior Assistant Grade 0 0 0 Assistant Grade 0 0 0 Subtotal 205,636 207,692 209,769 Ungraded 93 93 93 Total permanent positions 318 318 318 Total positions, end of year 417 417 417 Total full-time equivalent (FTE) employment, end of year 394 394 394 Average ES salary 177,884 179,663 181,460 Average GM/GS grade 12.4 12.5 12.5 Average GM/GS salary 106,681 107,748 108,825 Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
Major Changes in the Fiscal Year 2015 President’s Budget Request
The total change for the FY 2015 President’s Budget for NIDA, which is $7.514 million more than the FY 2014 Enacted level, for a total of $1,023.628 million results in no major changes.
Budget Graphs
History of Budget Authority and FTEs:
Distribution by Mechanism (dollars in thousands):
Change by Selected Mechanism:
Justification of Budget Request
National Institute on Drug Abuse
Authorizing Legislation: Section 301 and Title IV of the Public Health Service Act, as amended.
| FY 2013 Actual | FY 2014 Enacted | FY 2015 President's Budget | FY 2015 +/- FY 2014 | |
|---|---|---|---|---|
| BA | $992,232,176 | $1,015,754,000 | $1,023,268,000 | +$7,514,000 |
| FTE | 394 | 394 | 394 | 0 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Director's Overview
The impact of substance abuse in this country is daunting: the economic toll alone is estimated to exceed $600 billion a year in health care, crime-related, and productivity losses1. NIDA strives to invest in genetics, neuroscience, pharmacotherapy, and behavioral and health services research to develop new strategies for preventing and treating substance abuse and addiction.
Today’s Basic Science for Tomorrow’s Breakthroughs
There is a fundamental need to enhance our understanding of the multiple processes that contribute to human behavior and how their disruption can lead to addiction. A more detailed and personalized account of them will lead to more effective and precise interventions to prevent and treat this complex brain disorder.
In this context, and largely thanks to recent technological developments, we have made important advances along the axis linking genes with behavior. As a result, we now have an unprecedented capacity to screen for thousands of genetic variations and catalogue how they modulate abuse/addiction risk by influencing brain maturation, its neural architecture, and behavioral patterns. For example, NIDA supported researchers undertook a large genome-wide association study in which more than 8,000 research participants were genotyped on more than 500,000 single nucleotide polymorphism (SNP) markers. The first results of this effort identified 13 SNPs as potential candidates for variants associated with the brain’s ability to inhibit prepotent or inappropriate behaviors, a key marker of addiction vulnerability2.
However, it is now clear that the mere identification of vulnerability genes is only the beginning. By using such genome and, increasingly, whole individual sequence analysis to zero in on the genes that modulate risk, addiction researchers are also advancing their understanding of how environmental factors (e.g., parental style, drug exposure) can affect the expression of those genes to either strengthen or weaken behavioral patterns through epigenetic changes that do not change their sequence. A good example is the recent finding that exposure of adolescent rodents to nicotine causes epigenetic changes on the Fos B gene, which in turn sensitize brain circuits to the behavioral effects of cocaine administration3. The systematic identification of genetic, environmental, and neurocircuitry variations that modulate abuse/addiction risk is bound to revolutionize our prevention and treatment capacities.
Yet, despite the recent stunning progress in the neurosciences, many of the biggest questions about the human brain remain unexplored, largely due to a lack in ability to explore how individual cells and complex neural circuits interact in both time and space. This is why NIDA is excited to partner with other NIH Institutes in support of the President’s Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative that is focused on building a new arsenal of tools and technologies for helping scientists unlock the mysteries of the brain Working in partnership with other NIH institutes along with the Defense Advanced Research Projects Agency and the National Science Foundation, discoveries and innovations that come out of this Initiative will help the economy create jobs, make high technology solutions available to more people, and attract more young people to science.
All of these efforts are poised to usher in a new era in which neuroscience and technological advances will increasingly transform prevention interventions. Ongoing investigations to strengthen circuits (e.g., self-control), whose suboptimal functioning increase substance use risk 4,5, or to design health promoting messages better attuned to the unique neurobehavioral profile of young people6 are perfect examples of this true paradigm shift.
Big Opportunities in Big Data
Big data sets are essential platforms for the analysis of complex systems in genetics and epigenetics, proteomics, brain imaging and clinical science. For example, vast amounts of data are being produced by the overlaying of structural and functional brain imaging information that links the molecular and cellular data with the expression of higher-level brain function. A prime example is the new fMRI-based approach to generating images of the functional connectivity (FC) among brain regions in the absence of any specific task, the so called resting state (rs) FC. This technique offers a powerful window into circuit-level functions that may generate behavioral responses underlying vulnerability or a diseased state. Open access to such massive databases could lead to the identification of biomarkers of psychiatric illness risk, trajectory, and treatment response that will propel the field toward an unprecedented level of predictiveness and precision.
Similarly, in collaboration with the NIH Pain Consortium, NIDA is helping support the development of a free, open source, centralized national pain registry for tracking chronic pain sufferers and their self-reported outcomes over time. Combined with concerted efforts in the pharmacogenomics of prescription opiates, pain registries are poised to help us maximize the effectiveness of pain treatments while minimizing the likelihood of prescription opiate abuse and addiction. However, the analysis, interpretation, and quality control of big data sets present formidable challenges that will require the establishment of new infrastructure, training paradigms, interdisciplinary teams, and a culture change that reflects the importance of open access to data.
Nurturing Talent and Innovation
NIDA currently supports a great deal of innovative research on drug addiction and related health problems such as pain and HIV/AIDS and will continue to be at the forefront of training the next generation of innovative researchers. The 6-year old Avant-Garde award is a good example of a program that stimulates high–impact research that could lead to groundbreaking opportunities for the prevention and treatment of HIV/AIDS in drug abusers. NIDA is now crafting a new kind of award, which would blend NIH’s Pioneer and New Innovator award mechanisms. This new opportunity, called “AVENIR” awards, is designed to attract creative young investigators into HIV/drug abuse public health research.
Another example in this area are NIDA’s Cutting-Edge Basic Research Awards (CEBRA), designed to foster highly innovative or conceptually creative research that advances our understanding of drug abuse and addiction. The latest results of this effort include three independent studies exploring the potential benefits of neurofeedback training, transcranial magnetic stimulation, and meditation for facilitating smoking cessation. The latter study, showing that meditation improves the function of brain areas related to self-regulation, is of particular interest because it highlights the connection between seeking and rewarding creativity and the creation of opportunities to examine remediation of mental disorders in a completely new light.
Conclusion
The field of addiction research continues to benefit from the explosion in genetic knowledge, the advent of precise technologies to probe neuronal circuits, and the emergence of openly accessible big data platforms. NIDA’s research is strategically poised to take full advantage of these and other emerging opportunities to develop the knowledge base that can be used in the real world to reduce drug use in this country.
Overall Budget Policy: The FY 2015 President’s Budget request for NIDA is $1,023.268 million, an increase of $7.514 million or 0.7 percent above the FY 2014 Enacted level. Research priorities include those that position the Institute to advance the development of medications, taking advantage of innovative genetics tools and technologies, and translating the results of evidence-based findings to improve drug abuse interventions and promote greater access to them worldwide. HIV prevention and treatment is another top NIDA research priority, including research on the interactions between HIV, substance abuse, and other comorbid psychiatric disorders, linking vulnerable populations to HIV prevention, testing, and treatment services, addressing HIV/AIDS-related health disparities, and integrating the treatment of substance abuse and HIV. Funds are included in competing RPGs to support the trans-NIH BRAIN Initiative.
Program Descriptions and Accomplishments
Epidemiology, Services, and Prevention Research: This NIDA Division supports integrated approaches to understand and address the interactions between individuals and environments that contribute to drug abuse-related problems. It supports large surveys (e.g., the annual Monitoring the Future Survey, which tracks drug use and related attitudes among teens) and surveillance networks (e.g., the Community Epidemiology Work Group) to monitor drug-related issues and trends locally and nationally. Program efforts help identify substance abuse trends locally, nationally, and internationally; guide development of responsive interventions for a variety of populations; and encourage optimal service delivery in real-world settings. For example, factors associated with marijuana use have been undergoing dramatic changes. The potency, sources, availability, public perception, and legal status are significantly different than when marijuana use became a national issue more than 40 years ago. NIDA plans to support research to better understand the longer-term outcomes resulting from these changes, such as trends in use, harm perception, clinical/social consequences, brain development, educational outcomes, and market/demographic variables, particularly for adolescents and young adults. Such knowledge can be then used to inform policy, the public, and to improve prevention and treatment interventions.
Budget Policy: The FY 2015 President’s Budget estimate for this program is $253.817 million, an increase of $1.800 million or 0.7 percent over the FY 2014 Enacted level.
Basic and Clinical Neuroscience: The Basic and Clinical Neuroscience programs work together to expand understanding of the neurobiological, genetic/epigenetic, and behavioral factors that underlie drug abuse and addiction. Specifically, they examine which variables influence risk of drug abuse, addiction, and drug-related disorders; how addiction works in the brain, including the effects of drugs on the expression or silencing of genes; and how resultant changes affect brain function and consequent behaviors. Collectively, this research provides critical information to develop and test novel prevention and treatment interventions for drug abuse and addiction. For example, as mentioned above, a pressing research priority that has recently emerged due to the rapidly changing political and legal landscape surrounding marijuana, is the need to improve our understanding of the role of the endocannabinoid system in brain development, function and activity and the impact of marijuana use on these processes (particularly among young people). These programs also support fundamental research to better understand brain function. For example, our knowledge of the various mechanisms the brain uses to fuel its operations (i.e., brain energetics) is surprisingly limited. Energy utilization patterns in the brain enable and shape all mental and behavioral activities, both normal and pathological. Brain energy utilization (and thus behavior) is profoundly affected by environmental conditions, such as diet, stress and exposure to drugs of abuse. NIDA is, therefore, soliciting grant applications to improve our basic understanding of the molecular mechanisms whereby chronic exposure to drugs of abuse impacts brain energetics. Successful projects are poised to identify new molecular targets that could be harnessed to design novel medications and/or behavioral treatments for addiction.
Budget Policy: The FY 2015 President’s Budget request for this program is $440.720 million, an increase of $2.599 million or 0.6 percent over the FY 2014 Enacted level.
Program Portrait: Better pain management: a major goal of addiction research
FY 2014 Level: $42.4 million
FY 2015 Level: $42.7 million
Change: +$0.3 million
Pain management is an important component of high-quality compassionate medical care. Opioid analgesics are among the most effective and thus popular classes of drugs indicated for pain treatment. Unfortunately, the benefits of long-term opioid analgesic treatment are accompanied by significant risk of developing drug tolerance (and the need for escalating doses) and hyperalgesia (increased pain sensitivity). In addition, for persons with risk factors for addiction, exposure to potentially rewarding substances, like opioid analgesics, may reinforce drug-taking behavior. These are intrinsic liabilities of opiate analgesics that clearly increase the risk for diversion, abuse, addiction and overdose.
NIDA recognizes it has a critical role in ensuring the availability of safe and efficacious chronic pain management options while minimizing risk of abuse. This is why we are committed to supporting research designed to better predict who is at risk of addiction and to develop new classes of effective, nonaddicting pain medications. A good example of researchers’ attempts to identify predictors and markers of pain is the recent study in which MRI scans were used to “visualize” the telltale signs of physical pain in the brain and, for the first time, measure its intensity and tell whether a drug was relieving it (Wager at al, 2013). Although preliminary, these results are exciting because having a reliable signature (biomarker) for pain opens the door to a host of research and treatment opportunities, like an increased ability to define who needs opioid and how much.
One of the promising objects of study towards the development of non-opioid pain analgesics is a chemical called resiniferatoxin (RTX), which selectively kills overactive pain fibers in the spinal cord. NIDA helped to obtain clearance from the Food and Drug Administration (FDA) to begin clinical studies of RTX. Initial trials at the National Institutes of Health (NIH) Clinical Center have shown remarkable efficacy in the reduction of severe cancer pain.
Parallel to the efforts designed to develop pain medications with reduced abuse and addiction liability are the efforts that strive to minimize the risk of overdose with existing medications. It is worth highlighting a partnership established between NIDA and Lightlake Therapeutics Inc., to develop an intranasal delivery system of naloxone (an opioid receptor blocker that can rapidly reverse the overdose of prescription and illicit opioids) , which could greatly expand its availability and use in preventing opioid-related deaths, a public health problem of epidemic proportion in the U.S. Finally, NIDA is also pursuing non-pharmacological approaches to tackling this epidemic. Recognizing that when abused, even a single large dose can cause severe respiratory depression (and death), NIDA has launched a new SBIR initiative to develop mobile devices for the rapid identification of respiratory distress in the wearer to facilitate the rapid delivery of overdose reversal medications.
Pharmacotherapies and Medical Consequences: This program area is responsible for medications development aimed at helping people recover from drug abuse and addiction and sustain abstinence. For example, to leverage NIDA resources this program is encouraging the formation of strategic alliances between collaborating organizations (such as academic institutions, pharmaceutical and biotechnology companies) with the common goal of advancing medications through the development pipeline toward Food and Drug Administration (FDA) approval in a timely manner. This Program also includes research to address the medical consequences of drug abuse and addiction, including infectious diseases such as Hepatitis C virus (HCV) and HIV. Because of the high co-occurrence of substance abuse and infectious diseases, infectious disease specialists have a role to play in ensuring that their HIV+/HCV+ patients receive treatment for their substance-use disorders. NIDA plans to support research to address this critical gap by understanding both the barriers to and opportunities for engaging infectious disease specialists in implementing screening, brief intervention, and referral to treatment in their practices.
Budget Policy: The FY 2015 President’s Budget request for this program is $133.025 million, an increase of $1.128 million or 0.9 percent over the FY 2014 Enacted level.
Program Portrait: Medications development
FY 2014 Level: $78.6 million
FY 2015 Level: $79.2 million
Change: +$0.6 million
Next-generation pharmaceuticals will surely take advantage of new technologies. Chief among these are our current approaches to develop viable immunotherapeutic or biologic (e.g., bioengineered enzymes) approaches for treating addiction. The goal of this active area of research is the development of safe and effective vaccines or antibodies that target specific drugs, like nicotine, cocaine, and heroin, or drug combinations. If successful, immunotherapies--alone or in combination with other medications, behavioral treatments, or enzymatic approaches—stand to revolutionize how we treat, and maybe even someday prevent addiction.
Unlike conventional small molecule therapies, which target the neural pathways/receptors involved in drug addiction, immunotherapy targets the drug itself. The concept of addiction immunotherapies is exciting because the approach would stimulate the body to generate antibodies that bind specific drugs while they are still in the bloodstream, blocking their entry into the brain. The resulting reduction of reinforcing effects is expected to aid relapse prevention. The most advanced of these efforts relate to the development of a viable nicotine vaccine, which have established the necessary proof of principle (Hartmann-Boyce, et al. 2012). The more recent disappointing results of a Phase III clinical trial, however, suggests that further research and optimization is needed to bring the promise of this approach to clinical fruition.
Current efforts hinge on overcoming the relatively low antigenicity of these vaccines, identified as a key technical hurdle. To overcome this obstacle, a recent NIDA-supported preclinical study used an adeno-associated virus (AAV) to deliver a gene encoding a high-affinity anti-cocaine monoclonal antibody into mice. The introduction of this gene stimulated the animals to produce high levels of cocaine-specific antibodies, which prevented cocaine from entering the brain and rendered them impervious to cocaine's behavioral effects for at least four months after the injection (Rosenberg, et al. 2012).
An alternative approach involves the use of a highly efficient anti-drug enzyme to neutralize the drug while still in the bloodstream, keeping it from entering the brain. Recently, one group of researchers used the same adenovirus carrier system mentioned above to deliver a gene that encodes for an enhanced version of a naturally occurring cocaine-metabolizing enzyme called butyrylcholinesterase. Preliminary results in rodents showed that a single injection of a virus carrying this gene completely blocked cocaine's effects in the body and central nervous system for at least six months (Geng, et al 2013). NIDA has now established a strategic research partnership with Teva Pharmaceutical Industries to test the feasibility of delivering a bioengineered version of this enhanced and highly specific enzyme in humans.
Combined, these results suggest that using an adenovirus "shuttle" to deliver an efficient anti-cocaine antibody or cocaine metabolizing enzyme is a feasible approach and could be used to treat cocaine addiction. If successful, this strategy could also be applied to other drugs of abuse (e.g., methamphetamine, heroin) where the feasibility of producing high-affinity monoclonal antibodies has already been demonstrated. This type of approach would have other benefits in that it would obviate the need for patient adherence with frequent medication regimens or even multiple vaccine injections.
Clinical Trials Network: NIDA's National Drug Abuse Treatment Clinical Trials Network (CTN) comprises 13 research nodes and more than 240 individual community treatment programs in 38 States, plus the District of Columbia and Puerto Rico. The CTN develops and tests the feasibility and effectiveness of promising medications and behavioral treatment approaches for drug abuse and related disorders, such as comorbid mental health disorders and HIV, with diverse patient populations and community treatment providers. The CTN is currently at the final stage of completing (1) a multi-site study to evaluate the effect of Screening, Brief Intervention, and Referral to Treatment (SBIRT) in emergency departments on substance use and substance-related outcomes, (2) a trial of the safety and effectiveness of Suboxone (buprenorphine) plus Vivitrol (extended-release naltrexone) for the treatment of cocaine addiction in patients also abusing opioids, and (3) a randomized trial evaluating safety and preliminary efficacy of buspirone for relapse-prevention in patients with cocaine addiction. Ongoing studies are evaluating (1) the effect of contingency management on treatment engagement of HIV-infected drug users, (2) comparison of Vivitrol to Suboxone for patients addicted to heroin or other opioids, including prescription pain relievers, (3) N-acetylcysteine for treatment of marijuana addiction, (4) combination therapy with Vivitrol plus Wellbutrin (bupropion) for treatment of methamphetamine addiction, and (5) Vivitrol for HIV positive opioid users in HIV settings.
Budget Policy: The FY 2015 President’s Budget request for this program is $45.508 million, an increase of $0.500 million or 1.1 percent over the FY 2014 Enacted level.
Intramural Research Program (IRP): The Intramural program performs cutting edge research within a coordinated multidisciplinary framework. The IRP attempts to (1) elucidate the nature of the addictive process; (2) determine the potential use of emerging new therapies for substance abuse, both pharmacological and psychosocial; and (3) establish the long-term consequences of drugs of abuse on systems and organs, with particular emphasis on the brain and its development, maturation, function, and structure.
A prime example of the unique role the IRP plays in furthering substance abuse research is the recently established Designer Drug Research Unit (DDRU), created in response to the worldwide epidemic of synthetic drug abuse. Synthetic drugs are marketed as safe, cheap and legal alternatives to illicit drugs like marijuana, cocaine and ecstasy. However, they can produce serious cardiovascular and neurological side effects that require emergency medical care and can be fatal. Many popular designer drugs have been rendered illegal by regulatory control, but new replacement analogs are flooding the marketplace at an alarming rate. The NIDA IRP is uniquely poised to respond to this public health crisis by collecting, analyzing and disseminating current information about the pharmacology and toxicology of newly emerging designer drugs. The IRP also works collaboratively with NIDA’s Extramural Division of Pharmacotherapies and Medical Consequences of Drug Abuse to identify potential targets for addiction medications, an approach that should speed up the progress of selected targets along the NIDA medications development pipeline. In addition, NIDA and NIAAA together have made significant progress at integrating their intramural research programs in substance use, abuse, and addiction, including the appointment of a single Clinical Director for NIAAA and NIDA and the establishment of a joint genetics Intramural Research Program and a common optogenetics lab.
Budget Policy: The FY 2015 President’s Budget request for this program is $88.180 million, an increase of $0.873 million or 1.0 percent over the FY 2014 Enacted level.
Research Management and Support (RMS): RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. Additionally, the functions of RMS encompass strategic planning, coordination, and evaluation of NIDA's programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public. NIDA currently oversees more than 1,800 research grants and more than 190 research and development contracts. In addition to the infrastructure required to support research and training, NIDA also strives to educate the public about drug abuse and addiction and to raise awareness of the science addressing it.
Adolescents are a key target for NIDA’s outreach efforts. NIDA created National Drug Facts Week (NDFW), a weeklong health observance event held annually at the end of January during which teens and scientists connect at local events to discuss the scientific facts about drug abuse and addiction. In 2013, more than 500 events were held reaching all 50 states. Promotional media activities surrounding the week reached more than 71 million people. Another key audience is health care professionals. In October 2012, the Office of National Drug Control Policy (ONDCP) and NIDA launched two online continuing medical education courses—one focused on safe prescribing for pain, the other on managing patients who abuse prescription opioids—in partnership with Medscape. To date, these courses have been completed more than 60,000 times for credit, and more than 100,000 times not-for-credit. In 2014, NIDA will also publish a new NIDA Principles of Effective Treatment for Adolescents, intended to provide parents, referring clinicians, treatment practitioners, youth, and others with an evidence-based resource to the principles of effective substance abuse treatment for youth.
Budget Policy: The FY 2015 President’s Budget request for this mechanism is $62.018 million, an increase of $0.614 million or 1.0 percent over the FY 2014 Enacted level.
NIH Collaborative Activities: NIDA actively participates in a variety of trans-NIH activities.
For example:
- Common Fund - NIDA has a lead role for the Common Fund–supported Epigenomics Program and additionally will be administering a request for application (RFA) to support a data management center for the new Common Fund Extracellular RNA Communication Program.
- NIH Pain Consortium - NIDA is the lead on the NIH Pain Consortium Centers of Excellence in Pain Education (CoEPEs) to enhance patient outcomes by improving the education of health care professionals about pain and its treatment and the Pathways to Prevention Workshop (PPW) that will comprehensively review the literature on the effectiveness of opioids for the treatment of chronic pain.
- Collaborative Research on Addictions at NIH (CRAN) - NIDA, NIAAA, and the Tobacco Control Branch within NCI together lead the CRAN, which was established in 2013 to inject synergism and efficiency in the addiction field. To date, CRAN has released two funding announcements to promote collaborative research on addiction, and will be launching a new webpage to provide researchers and other stakeholders with funding information, news, and events as they emerge.
- NIDA is also integrally involved in the BRAIN (Basic Research through Advancing Innovative Neurotechnologies) Initiative – a trans-agency effort that includes NIH, the National Science Foundation, and Defense Advanced Research Projects Agency (DARPA). This initiative will build on recent successes in neuroscience to create and apply new tools for producing dynamic pictures of the brain that show how individual brain cells and complex neural circuits interact at the speed of thought. This new understanding will allow scientists to explore how the brain records, processes, uses, stores, and retrieves vast quantities of information, and shed light on the complex links between brain function and behavior. The information gleaned from this initiative will provide an unprecedented view of the complex neural changes that occur in addiction leading to new approaches for treating and preventing this devastating disease.
- In FY 2014, NIH will invest a total of $40 million to launch its part of the BRAIN Initiative. To ensure the Initiative’s success, NIH is requesting a total of $100 million in FY 2015 to advance the high priority research areas of the BRAIN Initiative, as outlined in its interim strategic plan. As one of the leaders of the BRAIN Initiative at NIH, NIDA is requesting an increase of $7 million in its budget to support these research priorities.
- NIH Blueprint – In addition to the BRAIN Initiative, NIDA is the lead for the NIH Blueprint–supported Institutional Training Grants on Computational Neuroscience and Neuroimaging, and the Neuroscience Information Framework (a dynamic inventory of Web-based neuroscience resources, data, and tools for scientists and students).
References
- Centers for Disease Control and Prevention. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5745a3.htm
Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009 Jun 27;373(9682):2223-33
National Drug Intelligence Center (2010). National Threat Assessment: The Economic Impact of Illicit Drug Use on American Society. Washington, DC: United States Department of Justice. - McGue, M. et al., 2013
- Levine, A. et al., 2011 (PDF, 811KB)
- Horrell, T. et al., 2010
- Muller, U. et al., 2012 (PDF, 136KB)
- Wang, A.et al., 2013