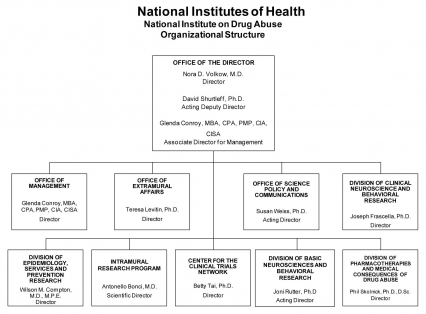
Organizational Chart
Appropriations Language
For carrying out section 301 and title IV of the PHS Act with respect to drug abuse, [$1,055,362,000] $1,054,001,000. (Department of Health and Human Services Appropriations Act, 2012.)
Tables
- Amounts Available for Obligation Table
- Budget Mechanism Table
- Summary of Changes Table
- Budget Authority by Activity Table
- Authorizing Legislation Table
- Appropriations History Table
- Budget Authority by Object Table
- Salaries and Expenses Table
- Details of Full-Time Equivalent Employment (FTEs) Table
- Detail of Positions Table
Major Changes in the Fiscal Year 2013 President's Budget Request
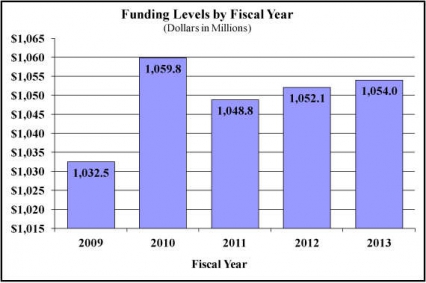
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2013 budget request for NIDA, which is $1.887 million greater than the FY 2012 Enacted level, for a total of $1,054.001 million.
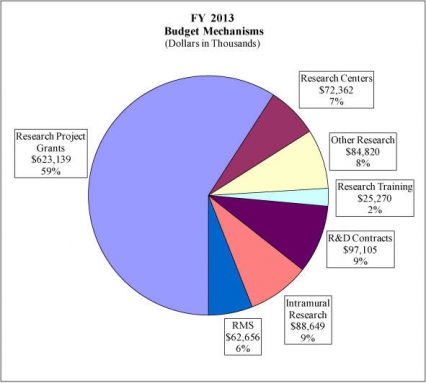
Research Project Grants (-$9.783 million; total $623.139 million): NIDA will support a total of 1,518 Research Project Grant (RPG) awards in FY 2013. Non-competing RPGs will decrease by 89 awards and $30.150 million. Competing RPGs will increase by 98 awards by $19.518 million. Within the total, research priorities include those that position the Institute to advance the development of medications, using innovative genetics tools and technologies, and translating the results of evidence-based findings to improve drug abuse interventions and promote greater access to them worldwide. NIDA will also continue to make the support of new and early stage investigators a priority. NIH budget policy for RPGs in FY 2013 discontinues inflationary allowances and reduces the average cost of noncompeting and competing RPGs by one percent below the FY 2012 level.
Research Training (+$0.225 million; total $25.270 million): NIDA will continue its support for the research training program by providing an increase of $225 thousand to fund a two percent increase in average stipend levels.
Intramural Research (+$0.791 million; total $88.649 million): The request will help offset the cost of other program increases. NIDA will work to identify areas of potential savings within the Intramural Research Program that will allow the institute to continue to achieve its program goals and accomplishments.
Research Management and Support (+$0.559 million; total $62.656 million): NIDA oversees over 1,800 research grants, more that 500 full-time training positions, and almost 200 research and development contracts. The increase will be used to partially offset the increases associated with cost increases necessary to provide for the effective administrative, planning and evaluation, public information and communication, and scientific leadership of the institute.
Budget Graphs
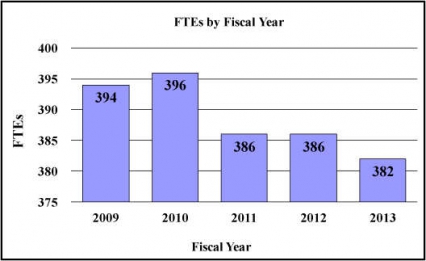
History of Budget Authority and FTEs:
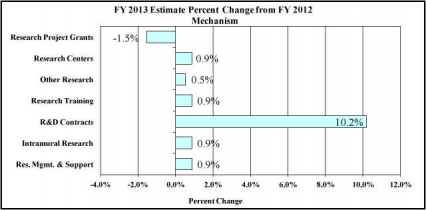
Distribution by Mechanism:
Change in Selected Mechanisms:
Justification of Budget Request
National Institute on Drug Abuse
Authorizing Legistlation: Section 301 and title IV of the Public Health Service Act, as amended.
| FY 2011 Actual | FY 2012 Enacted | FY 2013 President's Budget | FY 2013 +/- FY 2012 | |
|---|---|---|---|---|
| BA | 1,048,776,000 | 1,052,114,000 | 1,054,001,000 | 1,887,000 |
| FTE | 386 | 386 | 382 | -4 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Director’s Overview
Drug abuse and addiction present tenacious public health challenges, for although preventable, they are also persistent—bringing devastating consequences to individuals, families, and all of society. The good news is that the powerful tools and the detailed knowledge produced by modern neuroscience provide extraordinary opportunities to help solve these problems. The National Institute on Drug Abuse (NIDA) will continue to leverage its scientific leadership in this country and globally to (1) achieve a better understanding of substance abuse and addiction risk and the consequences of substance use disorders (SUDs) and (2) develop increasingly effective ways to prevent and treat both. The following themes and initiatives play prominent roles in NIDA’s strategic approach to tackling SUDs and their diverse medical and social consequences, in terms of legal (nicotine, prescription drugs, inhalants) and illegal drugs (cocaine, heroin, methamphetamine, marijuana).
Investments in Basic Research
Rapid technological advances of the past decade have enabled the various sub-disciplines within neuroscience to become increasingly intertwined and thus more capable of ushering progress toward understanding the complexity of the human brain and how drugs affect it. Recent scientific discoveries reveal the involvement of multiple brain circuits and neurotransmitters in addiction that go beyond the brain’s pleasure pathways (or reward system) to affect learning, memory, executive function (e.g., decision-making, self-control), and emotional reactivity. These discoveries offer potential new targets for prevention and treatment strategies. To realize fully the translational potential of this ongoing scientific revolution, NIDA is committed to the development of next-generation methods and tools capable of capturing and analyzing the vast and diverse emergent datasets containing actionable information on everything from genetics to social networks to neuroimaging.
Accelerating Discovery through Technology
Technical advances continue to transform research and clinical practice. NIDA is looking forward to harnessing complete genome and “deep” sequencing capabilities to uncover genetic information at the highest level of detail. These techniques, poised to expose rare genetic variants, are beginning to merge with a growing portfolio of epigenetic initiatives to explain how environmental factors (e.g., chronic stress), biological processes (e.g., brain development) and exposure to various neurological insults (e.g., exposure to drugs of abuse during fetal or later brain development) can alter the expression of specific genes that influence brain organization and function and how this in turn protects or facilitates expression of SUDs.
The overlaying of structural, neurochemical, and functional brain imaging information (for both the healthy state and during the pathological state of addiction) will further accelerate discovery by linking molecular and cellular data with human behavior. For example, a new functional magnetic resonance imaging (fMRI)-based approach can probe the resting brain (i.e., one not performing any specific task) to illuminate circuit-level functions that may prompt behavioral responses, including those related to diseased states or vulnerability. Individual differences found in these images of resting brain functional connectivity could provide useful biomarkers (neural signatures) of illness risk, course, and treatment response. Integrating data across all levels of inquiry will help to identify new targets for the development of addiction medications, including those capable of selectively turning genes “on and off” in the affected brain circuits to minimize or even reverse a drug-induced dysfunction.
Advancing Translational Sciences
Our greater ability to identify gene variants, epigenetic events, and brain imaging markers that correlate with or contribute to addiction vulnerability offers exciting opportunities for the development of medications to treat drug addiction. The pharmaceutical industry’s limited investment in addiction medications, due largely to stigma and lack of financial incentive, has made medications development a high priority for the institute.
Ultimately, success in translating NIDA’s research results into practice will depend on the extent to which this research leads to measurable improvements in the Nation’s health, which in turn depends on better healthcare delivery. Thus, implementation research to foster adoption of proven prevention and treatment strategies for drug abuse and its related health consequences represents a critical investment for NIDA towards improving public health. This strategy may be particularly useful in identifying barriers to implementation and in testing possible approaches to counter them. For example, NIDA is supporting research on how best to integrate screening, brief intervention, and referral to treatment into primary care and emergency room settings to identify substance related problems early on and prevent their escalation, or to better link those who need treatment with appropriate healthcare services.
Another critical focus of NIDA-supported research is the intersection of HIV and drug abuse, which have been intertwined since the start of the HIV epidemic, both through injection and non-injection drug use. One challenge is the lack of access to drug abuse and HIV treatment within those systems where drug abusers find themselves, including the criminal justice system, social services programs, clinical or other settings. To reach vulnerable groups, NIDA is testing the “seek, test, treat, and retain” (STTR) model for identifying and addressing HIV in diverse environments, including U.S. urban areas with high rates of injection drug use and in regions of the world where drug abuse is a prominent vector for HIV transmission. An overarching goal is to create the infrastructure and linkages needed to implement STTR and to integrate treatment for substance abuse and HIV, generating a large public health impact by reducing the prevalence and consequences of both.
Investing in the Next Generation of Scientists and Ideas
NIDA’s long-term goals can only be achieved by recruiting and retaining the brightest scientific minds. Thus, NIDA supports multiple training initiatives at various career levels and areas of need. Emerging priorities, like the need to tackle complex brain bioinformatics, have exposed a daunting lack of methods for probing increasingly complex systems. Success in this arena will hinge on our ability to train a new generation of scientists to create the language, tools, expertise, and infrastructure needed for researchers and clinicians to harmonize and analyze massive bits of data so that clinically useful information can be retrieved. Examples of such efforts include the multi-NIH Institute training programs in which NIDA participates, such as those focused on computational neuroscience, which strive to engage students early in a field needing innovation and expertise.
Conclusion. NIDA's philosophy and portfolio reflect a comprehensive, integrated, and nimble approach. It is one that takes full advantage of scientific advances to transform how we prevent and treat drug abuse and addiction (and related health consequences) in this country and abroad.
Overall Budget Policy: The FY 2013 President’s Budget request for NIDA is $1,054.001 million, an increase of $1.887 million or 0.18 percent from the FY 2012 Enacted level. Research priorities include those that position the Institute to advance the development of medications, using innovative genetics tools and technologies, and translating the results of evidence-based findings to improve drug abuse interventions and promote greater access to them worldwide. NIDA will also continue to make the support of new and early stage investigators a priority. Funds are included in R&D contracts to support trans-NIH initiatives, such as the Basic Behavioral and Social Sciences Opportunity Network (OppNet).
Program Descriptions and Accomplishments
Basic and Clinical Neuroscience: The Basic and Clinical neuroscience programs work together to expand our understanding of the neurobiological, genetic/epigenetic, and behavioral factors that underlie drug abuse and addiction. Specifically, they examine which variables influence risk of drug abuse, addiction, and drug-related disorders; how addiction works in the brain, including the effects of drugs on the expression or silencing of genes; and how resultant changes affect brain function and consequent behaviors. Collectively, this research provides critical information to develop and test novel prevention and treatment interventions for drug abuse and addiction. Continually evolving genetics and epigenetic research, coupled with rich data sets generated by burgeoning proteomics/metabolomics and brain imaging tools (e.g., optical imaging, fMRI, positron emission tomography), will help answer key questions such as how genes linked to addiction contribute to brain function and response to drugs of abuse; how orchestrated genetic networks drive complex, adaptive brain function; and how environmental stimuli can interact with those networks, producing epigenetic changes that upset their balance. New research in animal models offer an intriguing yet disturbing view of the possible longevity of such changes, affecting not only the individuals exposed, but also their offspring, and even the subsequent generation (i.e., transgenerational effects of drugs). Recent studies have begun to reveal how exposure to drugs can lead to epigenetic changes in the germline, or reproductive cells that can be transmitted across multiple generations through both maternal and paternal lines.
Program Portrait: Functional Connectivity/Biomarkers
FY 2012 Level: $11.500 million
FY 2013 Level: $11.500 million
Difference: --
NIDA is supporting research designed to elucidate neural correlates of risk and protection that influence brain development and substance use trajectories. For example, using functional magnetic resonance imaging (fMRI), the brain can be scanned in its resting state (rs) to generate maps of regions that operate together (i.e., functionally connected). Recently, rsfMRI maps were created for 1,400 healthy volunteers from around the globe. The resulting “open access” dataset is poised to establish critical benchmarks against which researchers will be able to compare patients with brain disorders or identify those at greater risk for addiction and other psychiatric disorders based on telltale “signatures”. Such signatures, or “biomarkers,” could become the basis of new diagnostic approaches that allow for the early detection and/or monitoring of psychiatric disorders, including addiction. Funded studies focus on functional connectivity analyses using rsfMRI data collected from both humans and animals. Follow-on studies will help identify structural and functional changes in key areas of the brain—such as those implicated in regulating affect, impulse control, reward, and motivation—to uncover the effects of drug abuse on brain structure and function.
For example, one study of patients addicted to prescription opioids found that compared with non-addicted individuals, addicted patients showed structural alterations in the brain region called the amygdala and decreases in the functional connectivity of several brain regions implicated in the regulation of mood, impulse control, reward and motivation. Another rsfMRI study found that, compared to control individuals, the higher impulsivity score (a hallmark of addiction) recorded in heroin-addicted patients correlated with significant differences in the connectivity maps of impulsivity-related networks. Other functional connectivity research is illuminating the neural correlates of risk-related behaviors and individual personality traits. The results of these studies suggest that individual differences in attitudes toward risk-taking are reflected in the brain's functional architecture, a notion with profound implications for understanding why a person engages in risky behaviors. Such studies further our understanding of the neural underpinnings of addiction-related traits and behaviors, thus helping to identify biomarkers that could help tailor therapeutic interventions, as well as identify promising new treatments.
Budget Policy: The FY 2013 President’s Budget request for this program is $478.902 million, an increase of $0.284 million and 0.06 percent over the FY 2012 Enacted level.
Epidemiology, Services and Prevention Research: This program area supports integrated approaches to understand and address the interactions between individuals and environments that contribute to drug abuse-related problems. Large surveys and surveillance networks that monitor drug-related issues exemplify programs supported by this NIDA Division. Program efforts help identify substance abuse trends locally, nationally, and internationally; guide development of responsive interventions for a variety of populations; and encourage optimal service delivery in real-world settings. For example, NIDA will continue to support epidemiological studies to understand the scope of and underlying reasons for prescription drug abuse to inform prevention efforts and help tailor and evaluate evidence-based interventions (proven effective for other drugs of abuse) to prescription drug abuse. Another exemplary initiative seeks to achieve better integration of drug abuse prevention and treatment in primary care settings by encouraging studies to identify the most effective strategies (e.g., drug abuse screening) and service delivery models for achieving this goal. Such studies are both important and timely as the implementation of the Affordable Care Act stands to increase the number of people accessing substance abuse services.
Budget Policy: The FY 2013 President’s Budget request for this program is $245.978 million, an increase of $0.146 million and 0.06 percent over the FY 2012 Enacted level.
Pharmacotherapies and Medical Consequences: This program area is responsible for medications development aimed at helping people recover from drug abuse and addiction and sustain abstinence, and includes development of non-addictive pain medications. It capitalizes on research showing the involvement of different brain systems in drug abuse and addiction, beyond the pleasure pathway (reward system), to develop medications in response to a variety of newly defined targets (see Program Portrait below). To elicit crucial pharmaceutical interest and involvement, NIDA is also supporting the ongoing development of promising anti-addiction vaccines to derive better adjuvants and haptens (agents that increase the immune response). This includes vaccines against cocaine, heroin, and methamphetamine, as well as nicotine—e.g., a vaccine that does not require a pharmacological agent to be added to increase the immune response (i.e., it is “self-adjuvanting”). NIDA also continues to stimulate advances in medications development through its Translational Medications Avant-Garde Awards program, which supports innovative projects that are ready for clinical translation and can accelerate the development of new medications. Another new strategy is to support public-private partnerships to advance effective anti-smoking medications. The goal is to leverage the strengths of public (government agencies and institutes), non-profit (academia, NGOs, philanthropic institutions) and private sector entities to incentivize a steady flow of compounds that can more rapidly progress from “molecules to medicine.” This program area also seeks solutions addressing the medical consequences of drug abuse and addiction, including infectious diseases such as HIV.
Program Portrait: Extraordinary Opportunities in Medications Development for Addiction
FY 2012 Level: $98.600 million
FY 2013 Level: $99.000 million
Difference: $0.400 million
The development of addiction medications is a top research priority for NIDA. To attract pharmaceutical investment,NIDA has revamped its approach to medications development research to provide greater up-front support for a shorter period of time. This shift was prompted in part by the highly successful clinical trials of Probuphine—a buprenorphine medication implanted under the skin—which received fast funding from the American Reinvestment and Recovery Act and produced promising early results. Refocusing medications funding in this way allows projects to demonstrate results that, in turn, could lead to investment by the private sector.
Scientific opportunities that offer promise exist in the following research areas: genomics/epigenetics, neuroscience, and immunotherapies (e.g., “vaccines”). A compelling example is the discovery of a cluster of nicotinic acetylcholine receptor genes on chromosome 15 linked to nicotine dependence; specifically, the α5 nicotinic receptor gene within this cluster was identified as a potential medication target since it was found to affect nicotine withdrawal, a major triger of relapse in tobacco users. Another novel strategy comes from epigenetic studies showing biological changes involved in the transition from acute, controlled drug intake to compulsive administration. Such discoveries offer a completely new array of potential medication targets. In addition, advances in our knowledge of how drugs cause the neuroplastic changes that affect brain circuit functioning linked to reward, motivation, and self-control have expanded the scope of pharmacological strategies for interfering with addiction-related behaviors like compulsive drug seeking and use. Finally medications that target neuronal circuits common to multiple addictions (e.g., dampening stress reactivity, inhibiting conditioning) are also likely to benefit other disorders, such as post-traumatic stress disorder or obesity.
Having viable medications available to treat substance use disorders, so costly to our society, would help these conditions become recognized as medical disorders, reducing the associated stigma and facilitating the proper treatment of people suffering from this disease. Given the dearth of medications available to treat addiction (including nicotine addiction), support of medications development for this purpose will have a major impact on public health and the overall economy.
Budget Policy: The FY 2013 President’s Budget request for this program is $130.157 million, an increase of $0.078 million and 0.06 percent over the FY 2012 Enacted level.
Clinical Trials Network: NIDA's National Drug Abuse Treatment Clinical Trials Network (CTN), which now comprises 13 research nodes and more than 240 individual community treatment programs in 38 States, plus the District of Columbia and Puerto Rico. The CTN works to develop treatment protocols for drug abuse and addiction and related conditions, such as comorbid mental health disorders and HIV, testing the real-world effectiveness of promising medication and behavioral treatment approaches with diverse patient populations and community treatment providers. It also serves as a research and training platform to help NIDA respond to emerging public health threats. Since 1999, the CTN has increased adoption of research-based treatments at the community level. For example, CTN trials have shown that Suboxone (buprenorphine/naloxone) is a safe and effective treatment for opioid addiction in young adults and in those abusing prescription opioid pain relievers. Suboxone, which can be prescribed by qualified physicians in primary care, is an alternative to methadone that can expand the reach of effective drug abuse treatment for opioid addiction. Additionally, CTN trials showed that rapid HIV testing can be implemented in community treatment drug abuse centers, thus contributing to more comprehensive health care for drug abuse patients. The CTN is currently preparing for
a multisite study to evaluate web-delivery of evidence-based psychosocial treatment for substance use disorders, a study of the safety and effectiveness of buprenorphine plus naloxone for the treatment of cocaine addiction in patients addicted to both cocaine and opiates, and a study evaluating the impact of screening and brief intervention in emergency departments on substance use and substance-related outcomes.
Budget Policy: The FY 2013 President’s Budget request for this program is $47.659 million, an increase of $0.029 million and 0.06 percent over the FY 2012 Enacted level.
Intramural Research Program (IRP): The Intramural program performs cutting edge research within a coordinated multidisciplinary framework. The IRP attempts to (1) elucidate the nature of the addictive process; (2) determine the potential use of emerging new therapies for substance abuse, both pharmacological and psychosocial; and (3) establish the long-term consequences of drugs of abuse on systems and organs, with particular emphasis on the brain and its development, maturation, function, and structure. In addition, the IRP supports an HIV/AIDS Pathophysiology and Medications Discovery Program, which focuses on (1) how HIV or its products cross the blood-brain barrier, (2) how toxic compounds generated by HIV invade brain cells, and (3) the development of compounds to block the toxic effects of HIV on immune system cells. IRP investigators are working on varied potential targets for addiction medications, many of which are being pursued through collaborative efforts with NIDA’s extramural medications development program, an approach that should speed up the progress of selected targets along the NIDA medications development pipeline. For example, researchers at the IRP are exploring promising early findings for the medication +naloxone, an opiate blocker that selectively targets glia cells and not neurons. Recent findings suggest that +naloxone can mitigate the behavioral response to both morphine and cocaine as well as the sudden release of dopamine in the brain’s reward center. Future tests are planned to further evaluate the mechanisms involved, any possible adverse effects and toxicity of the compound, and its potential to treat addiction in humans.
Budget Policy: The FY 2013 President’s Budget request for this program is $88.649 million, an increase of $0.791 million and 0.90 percent over the FY 2012 Enacted Level.
Research Management and Support (RMS): RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. Additionally, the functions of RMS encompass strategic planning, coordination, and evaluation of NIDA's programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public. NIDA currently oversees more than 1,800 research grants and more than 170 research and development contracts. In addition to the infrastructure required to support research and training, NIDA also strives to educate the public about drug abuse and addiction and to raise awareness of the science addressing it. This year NIDAMED, NIDA’s physician outreach initiative that provides screening tools and other resources for use in primary care settings, expanded its activities to further break down some of the barriers preventing medical professionals from implementing drug abuse screening in their practices. A NIDA Quick Screen was added to NIDA’s drug abuse screening tool, providing a single screening question for physicians to use with their patients. Since it was launched in April 2009, the tool has been accessed about 18,000 times. Also part of NIDAMED’s outreach, NIDA launched the innovative Addiction Performance Project–a continuing medical education (CME) and continuing education (CE) program intended to remove the stigma of drug abuse in the health care system by promoting a dialogue between patients and physicians/nurses and engender empathy for patients with the disease. Another national health awareness campaign—this one directed at teens—is NIDA’s National Drug Facts Week (NDFW). NDFW is a spinoff of NIDA’s popular Drug Facts Chat Day, now in its 5th year. The second annual NDFW was a great success, with more than 130 events held in communities across the country helping students learn the scientific facts about drug abuse and addiction. In addition, the interactive Sara Bellum Blog, a key feature of NIDA’s innovative teen website that regularly shares science-based messages about drug use and health, won Silver in the Web Health Awards in spring 2011 for its appeal to teens, with people from various public health organizations asking to be guest bloggers.
Budget Policy: The FY 2013 budget estimate for this program is $62.656 million, an increase of $0.559 million and 0.90 percent over the FY 2012 Enacted level.
NIH Collaborative Activities: NIDA participates in a variety of activities supported through the Common Fund; the Basic Behavioral and Social Sciences Opportunity Network, or OppNet; and the Neuroscience Blueprint. Among these, NIDA has a lead role on (1) the Epigenomics-funded grants initiated under Roadmap; (2) an OppNet-supported RFA (DA-11-003) titled the Effects of the Social Environment on Health, which will fund research to investigate structural, behavioral, sociocultural, environmental, cognitive, emotional, and/or biological mechanisms by which the social environment affects health outcomes; and (3) the NIH Blueprint–supported Institutional Training Grants on Computational Neuroscience and Neuroimaging—Integrating First Principles and Applications.