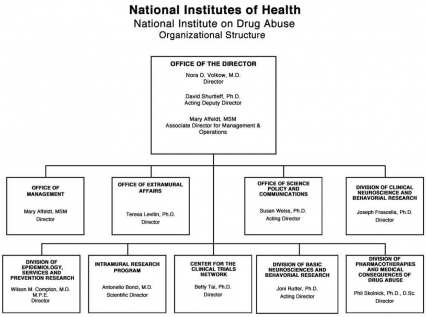
Organizational Chart
Appropriations Language
For carrying out section 301 and title IV of the Public Health Services Act with respect to drug abuse $1,080,018,000.
Tables
- Amounts Available for Obligation Table
- Appropriations History Table
- Authorizing Legislation Table
- Budget Authority by Activity Table
- Budget Authority by Object Table
- Budget Mechanism Table
- Detail of Full-Time Equivalent Employment (FTE) Table
- Detail of Positions Table
- New Positions Requested Table
- Salaries and Expenses Table
- Summary of Changes Table
Major Changes in the Fiscal Year 2012 Budget Request
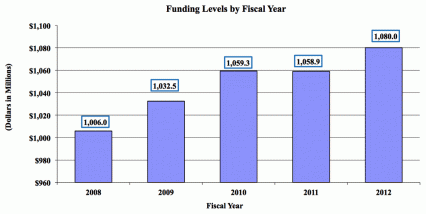
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2012 budget request for NIDA, which is $20.8 million greater than the FY 10 Estimate, for a total of $1.080 billion.
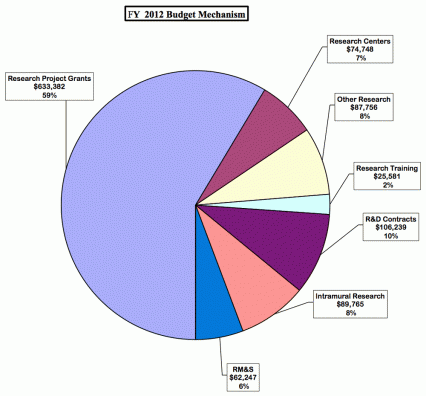
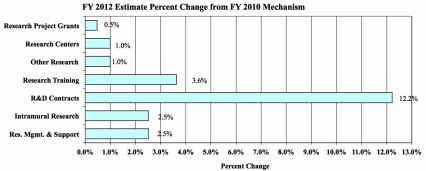
Research Project Grants (+$2.754 million; total $614.122 million): NIDA will support a total of 1,312 Research Project Grant (RPG) awards in FY 2012. Non-competing RPGs will decrease by 56 awards and increase by $7.2 million. Competing RPGs will decrease by 44 awards and decrease by $1.4 million. Within the total, NIDA will provide increases for the Director's scientific priorities, which include genomics and other high-throughput technologies, translational medicine, and initiatives that benefit health care reform and train new investigators.
Research Training (+$906 thousand; total $25.881 million): NIDA will continue its support for the research training program by providing an increase of $906 thousand to fund a four percent increase in average stipend levels.
Intramural Research (+$2.200 million; total $89.765 million): The request will help offset the cost of other program increases. NIDA will work to identify areas of potential savings within the Intramural Research Program that will allow the institute to continue to achieve its program goals and accomplishments.
Research Management and Support (+$1.527 million; total $62.247 million): NIDA oversees over 1,700 research grants, more that 500 full-time training positions, and almost 200 research and development contracts. The increase will be used to partially offset the increases associated with cost increases necessary to provide for the effective administrative, planning and evaluation, public information and communication, and scientific leadership of the institute.
Budget Graphs
History of Budget Authority and FTE's
Distribution by Mechanism
Change in Selected Mechanisms
Justification of Budget Request
Authorizing Legislation: Section 301 and title IV of the Public Health Service Act, as amended.
| FY 2010 Actual | FY 2011 Continuing Resolution | FY 2012 Budget Request | FY 2012 +/- 2010 | |
|---|---|---|---|---|
| BA | 1,059,266,000 | 1,058,947,000 | 1,080,018,000 | 20,752,000 |
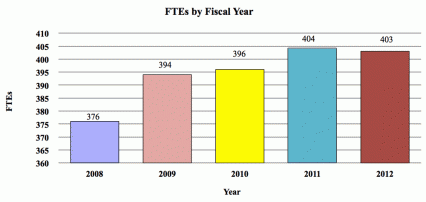
| FTE | 396 | 397 | 397 | +1 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Director's Overview
Drug addiction presents a disturbing paradox, for it is (1) a disease with a formidable power to destroy lives and shred the social fabric and yet (2) it is totally preventable. Knowledge is the foundation of the transformative agenda needed to strike at the heart of this stubborn and costly challenge. Thus, National Institute on Drug Abuse (NIDA) will continue to leverage its scientific leadership to achieve a better understanding of addiction risk, consequences, and solutions. The following initiatives play a prominent role in NIDA's strategic approach to reaching this goal.
The tools of progress.
Technological advances continue to revolutionize virtually every aspect of research and clinical practice. NIDA is looking forward to harnessing complete genome and "deep" sequencing capabilities, tools designed to uncover genetic information at the highest conceivable level of detail. This capability allows investigators to identify rare genetic variants beyond the reach of traditional genome-wide technologies. These efforts are beginning to merge with our growing portfolio of epigenetic initiatives designed to uncover how environmental factors (e.g. chronic stress), biological processes (e.g. brain development) and exposure to various brain toxins (e.g. drugs of abuse) can alter the expression of specific genes that influence the start or continuation of addictive behaviors. This combined approach is poised to reveal new targets for the development of addiction medications, including those capable of turning gene expression on or off selectively in the affected brain circuits so as to minimize or even reverse the drug-induced dysfunction. NIDA is also investing in a new fMRI-based approach to probe the resting brain and generate images of the functional connectivity (rsFC) among brain regions in the absence of any specific task being performed. The technique offers a powerful new window into circuit-level functions that may generate behavioral responses, including those related to diseased states or vulnerability. Thus, individual differences in rsFC could become useful biomarkers (neural signatures) of psychiatric illness risk and trajectory.
Evolving and disseminating better treatments.
NIDA remains focused on the goal of translating and deploying better addiction treatments and will steadfastly support the development of new medications while trying to compensate for the chronic lack of pharmaceutical investment in this area. Several advances are particularly noteworthy: Vivitrol, an extended-release form of naltrexone, has recently been approved for the treatment of opioid addiction.1 In contrast to methadone or buprenorphine, Vivitrol is not an opioid analogue, in that it does not function as a "replacement therapy" and so is more likely to gain acceptance by the treatment community. Another breakthrough is the development of immunotherapies, or "vaccines" for various addictions, which work by generating drug-specific antibodies that bind the drug while in the bloodstream and prevent its entry into the brain.2 Among current efforts, a promising nicotine vaccine has demonstrated success in clinical trials. The vaccine improves smoking quit rates and is now in phase III trials to evaluate abstinence at 12 months; final data is expected in early 2012.3,4 Finally, NIDA maintains a focus on harnessing novel treatment platforms, such as technologies for enhancing medication efficacy (e.g., strengthening cognition to improve outcomes) or for use as standalone treatments. Neurofeedback is one approach that may help patients control their drug cravings by allowing them to directly alter their brain responses through feedback obtained from viewing real-time images of their brain activity.5 Another noninvasive technology under study is transcranial magnetic stimulation (TMS), which stimulates specific brain regions with a set of magnetic pulses that can penetrate the skull through application to the scalp. Already used to treat several other mental illnesses, TMS may prove useful for enhancing brain circuits related to behavioral inhibition, the lack of which is a hallmark of addiction.6
Building the scientific foundation for reformative healthcare.
The success of NIDA's efforts will depend on the extent to which our research leads to measurable improvements in the Nation's health. Thus, conducting research to develop effective addiction medications and identify the best tools and technologies for improving the delivery of healthcare and its associated medical infrastructure represent critical aspects of our forward thinking. NIDA's initiatives in this area are geared toward the following:
- Expanding electronic medical records to include addiction treatment, and facilitating the use of clinical data sets.
- Validating and optimizing tools for screening and brief intervention, and increasing access to the healthcare system.
- Expanding our physician outreach education efforts, particularly those related to prescription drugs and their potential for diversion, misuse, and abuse.
- Deploying evidence-based, cost-effective treatment innovations (medications and behavioral treatments for drug abuse and addiction) into the criminal justice system to improve outcomes and reduce recidivism.
- Reaching high-risk and vulnerable groups (e.g, criminal justice populations) though assertive outreach to identify, test, and initiate HIV treatment for positive testers (Seek, Test, and Treat initiative), thereby helping prevent HIV transmission, incidence, and progression to AIDS.
- Conducting comparative effectiveness research among HIV+ drug abusers to garner better medication adherence using a combination of evidence-based approaches v. standard education.
Innovative investigators, fresh ideas.
Multiple advances in science and policy arenas have led to a natural excitement within both lay and research circles about research and clinical opportunities on the horizon. However, the prolonged economic downturn threatens the envisioned possibilities and demands a sustained commitment to cultivating the next generation of biomedical research scientists. To this end, NIDA supports multiple training initiatives at various career levels and areas of need (e.g., physician scientists, computational neuroscience, and medicinal chemists). Examples include multi-Institute training programs within the NIH Blueprint from which could issue innovative brain research on a variety of neurological/psychiatric conditions. To reach the next generation, NIDA also awards special prizes at the annual Intel International Science and Engineering Fair to high school students whose projects exemplify achievement in addiction science.
Conclusion
NIDA's portfolio reflects a comprehensive, integrated, and nimble approach aimed at taking full advantage of scientific advances and translating them into knowledge to transform the way we prevent and treat drug abuse and addiction and their related health consequences in this country and abroad.
Overall Budget Policy:
The FY 2012 request for NIDA is $1.080 billion, an increase of $20.8 million or + 2.0 percent over the FY 2010 enacted level. NIDA will continue to make the support of new and early stage investigators a priority. Research priorities include those that position the Institute to optimally benefit from scientific advances able to transform our progress in preventing and treating drug abuse and its consequences and thereby further NIDA's public health mission. NIDA will support research that uses innovative genetics tools and technologies, furthers development of promising medications, and that translates the results of evidence-based findings to improve drug abuse interventions and promote greater access to them worldwide. The Institute also seeks to maintain a balance between solicitations issued to the extramural community and funding made available to support investigator-initiated projects. Intramural research and research management and support receive increases to help offset the cost of pay and other costs. In addition, funds are included in R&D contracts to reflect NIDA's share of NIH-wide funding required to support several trans-NIH initiatives, such as the Therapies for Rare and Neglected Diseases program (TRND), the Basic Behavioral and Social Sciences Opportunity Network (OppNet), and support for a new synchrotron at the Brookhaven National Laboratory. For example, each IC that will benefit from the new synchrotron will provide funding to total NIH's commitment to support this new technology -- $10 million.
NIH will provide an increase of four percent for stipends levels under the Ruth L. Kirschstein National Research Service Award training program to continue efforts to attain the stipend levels recommended by the National Academy of Sciences. This will build on the two percent increase in stipend levels for FY 2011. Stipend levels were largely flat for several years, and the requested increase will help to sustain the development of a highly qualified biomedical research workforce.
Program Descriptions and Accomplishments
Basic and Clinical Neuroscience: Basic and Clinical neuroscience represent two programs in NIDA that work together to enlarge understanding of the neurobiological, genetic/epigenetic, and behavioral factors underlying drug abuse and addiction. Specifically, they examine the factors affecting increased risk and/or resilience to drug abuse, addiction, and drug-related disorders; the mechanisms of addiction; and the effects of drugs on the expression or silencing of genes in the brain, on brain development function and neurochemistry, and on how these changes relate to behavior. Collectively, this research provides the fundamental information to develop and communicate prevention and treatment interventions for drug abuse and addiction. One example is research that may yield new tools to facilitate more accurate screening for signs of chronic drug use in patients and to monitor treatment efficacy. A new functional magnetic resonance imaging (fMRI) application evaluates the brain at rest to generate functional connectivity maps, which show synchronized networks of neuronal activity comprising multiple brain regions. These maps present highly detailed features, which should include reliable signatures of disease risk that could spur the development of novel diagnostic instruments and/or approaches to help facilitate the early detection and/or monitoring of psychiatric disorders, including addiction.
Program Portrait: Genetics/Epigenetics of addiction
FY 2011 Level: [$123.466 million]
FY 2012 Level: [$125.812 million]
Why do some people become addicted, while others do not? Studies of identical twins have shown that as much as half of an individual's risk of becoming addicted to nicotine, alcohol, or other drugs depends on his or her genes. Pinning down the biological basis for this risk is an essential, albeit challenging goal of addiction research. Thousands of genes influence addiction risk and trajectory, the net effects of which are often suppressed or enhanced by environmental and developmental factors. Continuous advances in genomics research have yielded tools of unprecedented power to identify the contribution of genetic factors and to understand how external stimuli and circumstances influence their impact.
Much of the foundation we have today for the improved understanding of the genetics of addiction derives from Genome Wide Association Studies (GWAS), which have demonstrated a remarkable capacity to implicate both known and previously unknown disease contributing genes. Using GWAS, investigators have identified approximately 100 genes linked to drug abuse and addiction. These include variations in a gene cluster with a strong effect on nicotine dependence and related disease. These replicated findings are remarkable because they reveal promising new targets for next-generation medications for treating nicotine addiction and lung cancer. While we will continue to reap the benefits of GWAS for the foreseeable future, NIDA is looking toward harnessing newer tools, such as complete genome and "deep" sequencing capabilities to help uncover genetic information at the highest level of detail, allowing the identification of new or rare variants beyond the reach of traditional GWAS technology. Complementing these efforts, NIDA will continue to support epigenetics research to gain valuable insight into the mechanisms whereby the environment can influence genetic background or addiction propensity. To better understand how genes affect brain development and function and, in turn, influence behavior, NIDA is committed to facilitating the merging of genomic and brain imaging databases, which will yield more reliable connections between particular genotypes and abuse/addiction risk, and help to create new opportunities to hone prevention, diagnosis, and treatment approaches. To make all these exciting promises a reality, NIDA is involved in amassing the required informatics and technological infrastructure, working with other Institutes and disciplines to collate and harness the full potential of the expanding genetic datasets already being generated.
Budget Policy: The FY 2012 budget estimate for this program is $501.075 million, an increase of $9.191 million and 1.9 percent over the FY 2010 estimate.
Epidemiology, Services and Prevention Research: This major program area seeks to promote integrated approaches to understand and address the interactions between individuals and environments that contribute to the continuum of drug abuse-related problems. This NIDA division supports research and major data collection systems, as well as surveillance networks. Program efforts help identify substance abuse trends locally, nationally, and internationally; guide development of responsive interventions for a variety of populations; and encourage optimal service delivery in real-world settings. One exemplary intervention has shown that nurse home visits during pregnancy and the first 2 years of life have enduring beneficial effects on substance use, mental health, and academic achievement. Findings from a Memphis Nurse Family Partnership study showed significant intervention effects on participants at age 12; those visited by nurses compared to those in the control group had less tobacco, alcohol, and marijuana use, were less likely to have internalizing disorders (e.g., anxiety and depression), and scored higher on math and reading achievement tests during their first 6 years of education.
Program Portrait: Accessing hard-to-reach populations to improve HIV/AIDS outcomes here and abroad
FY 2011 Level: [$.700 million]
FY 2012 Level: [$10.000 million]
The Centers for Disease Control and Prevention estimates that 1 million people are living with HIV in this country, with 56,000 new cases occurring each year—a rate that has held stubbornly steady over the past decade.7 Internationally, the pandemic has been devastating in multiple regions of the world. Worldwide, approximately 33 million people are living with HIV, with 2.7 million new infections and 2 million deaths occurring each year.8 From the time it began, the HIV/AIDS epidemic has been closely linked with drug abuse and addiction, a linkage that the National Institute on Drug Abuse (NIDA) has judiciously pursued to better understand how best to prevent and treat these intertwined conditions, both here and abroad. NIDA-supported researchers have risen to this formidable challenge through strategies designed to (1) prevent HIV risk behaviors in substance abusers; (2) encourage early testing and initiation of highly active antiretroviral therapy (HAART) treatment, and (3) provide greater access to evidence-based drug abuse treatments, including medications, as a means of diminishing HIV spread. These strategies focus on high-risk populations, and are capable of preventing HIV transmission and decreasing incidence.
NIDA is supporting research, inspired by Dr. Julio Montaner—a pioneer and activist in HIV/AIDS research and a recipient of NIDA's Avant-Garde Award for innovative research approaches—to develop and test strategies to expand HAART coverage and thereby reduce viral load and HIV incidence at the population level. Known as "Seek, Test, Treat, and Retain," this initiative will identify individuals who have not recently been tested, and start them on HAART therapy if they test positive, monitoring their progress over time. Implemented in high-risk populations in this country and abroad, particularly in the criminal justice system or in regions of the world where the HIV scourge is driven by intravenous drug use, his initiative could catalyze a needed paradigm shift in how we approach HIV prevention and treatment, targeting hard-to-reach populations typically outside the treatment loop. This includes people with substance use disorders, frequently excluded from HIV treatment because of the misguided notion that they cannot be effectively treated with HAART.
The lack of access to HAART in regions of the world such as Eastern Europe and Central Asia—where the intertwined epidemics of injection drug use and HIV are fueling devastating disease and societal disintegration—is compounded by a resistance in some countries to adopting effective drug abuse treatments, particularly opioid agonist medications. Vivtrol, recently approved by the FDA for opioid dependence (see Portrait), could help change this situation. An injectable form of the opioid antagonist naltrexone, Vivitrol garnered impressive results in clinical trials in Russia, which could make it a key player in other countries where opiate replacement therapy is rejected. This includes China, where NIDA plans to expand the conduct of clinical trials of Vivitrol.
Budget Policy: The FY 2012 budget estimate for this program is $249.203 million, an increase of $4.572 million and 1.9 percent over the FY 2010 estimate.
Pharmacotherapies and Medical Consequences: This program area is responsible for medications development aimed at helping people recover from drug abuse and addiction and sustain abstinence. It capitalizes on research showing the involvement of different brain systems in drug abuse and addiction, beyond the dopamine system, to develop medications in response to a variety of newly defined targets. This program area also seeks solutions addressing the medical consequences of drug abuse and addiction, including infectious diseases such as HIV. New delivery mechanisms are being developed to improve treatment adherence, reduce diversion, and prevent relapse. For example, immunotherapies, or "vaccines," which induce production of antibodies to block specific drugs from entering the brain, can be effective for months following vaccination and help prevent relapse. Nicotine and cocaine vaccines are now undergoing testing in humans. Another example of a long-acting (depot) medication is Vivitrol, an injectable version of the opioid antagonist naltrexone, prescribed for alcohol addiction and now available to treat heroin/opioid addiction. NIDA supported the initial research to develop an injectable formulation of naltrexone as well as later "investigational new drug" IND-enabling studies by a pharmaceutical company, which helped Vivitrol receive FDA approval this year. Vivitrol is the first non-narcotic, non-addictive, extended release medication approved for the treatment of opioid dependence, marking an important turning point in our approach to treatment.
Program Portrait: Medications advances for drug abuse and addiction: new formulations, better treatments
FY 2010 Level: [$99.208 million]
FY 2012 Level: [$101.093 million]
Recent scientific discoveries have bolstered NIDA's commitment to the development of potential medications, which are now more important than ever. Pharmaceutical companies, historically disinclined to invest in this area due to perceived stigma and financial disincentives, are now retreating even further, reducing their investment overall in psychotherapeutic medications. This situation makes it even more critical for NIDA to continue its medications push, building on promising discoveries and taking advantage of new targets and alternative delivery systems that herald a new era in addiction treatment.
Recent progress in this area includes novel long-acting (or depot) formulations, and the innovative approach of developing vaccines to produce drug-specific antibodies that prevent the drugs from entering the brain and exerting their psychoactive effects. Work is ongoing for cocaine and heroin, but the most advanced of these is a nicotine vaccine (NicVax), currently in Phase III clinical trials.
Innovative long-acting formulations are also being tested for opioid addiction, which afflicts many people in this country, including approximately 810,000 addicted to heroin9 and about 1.9 million abusing or addicted to prescription pain relievers.10 Although medications do exist for opioid addiction, treatment is frequently complicated by difficulties related to treatment access and adherence, and to diversion of opioid agonist medications, especially for methadone. Two recent developments signal important treatment advances. First, Vivitrol, an injectable form of the opioid antagonist naltrexone (already available as a treatment for alcoholism), recently received approval for treating opioid addiction based on impressive results from a study conducted in Russia among people addicted to heroin. Vivitrol produced a median 90% rate of opioid-free urines versus 35% among controls; a 50% reduction in opioid craving versus no change for placebo; and a 75% longer retention in treatment.11 This could make it a key player in situations where opiate replacement therapy is rejected or patients are hard to reach, including within the criminal justice system, where NIDA is currently studying its effectiveness.
If successful, Vivitrol could also make an impact on public health in Eastern Europe and Central Asia, where the intertwined epidemics of injection drug use and HIV are fueling devastating disease and societal disintegration. Second, an ARRA-funded NIDA comparative effectiveness study is testing an alternative method for delivering buprenorphine, also used to treat opioid addiction, comparing a subcutaneous (under the skin) implant with a daily sublingual (under the tongue) tablet formulation currently in use. The implantable formulation, Probuphine, enables continuous delivery of the medication for 6 months after a single treatment, which may prove more effective than the tablet form by preventing poor treatment adherence and medication diversion. Probuphine is currently in Phase 3 clinical trials, with clinical recruitment ahead of schedule.
Budget Policy: The FY 2012 budget estimate for this program is $132.085 million, an increase of $2.424 million and 1.9 percent over the FY 2010 estimate.
Clinical Trials Network: NIDA's National Drug Abuse Treatment Clinical Trials Network (CTN), which now comprises 16 research nodes and more than 240 individual community treatment programs, serves 34 States, plus the District of Columbia and Puerto Rico. The CTN works to develop treatment protocols for drug abuse and addiction and related conditions, such as comorbid mental health disorders and HIV, testing the real-world effectiveness of promising medication and behavioral treatment approaches with diverse patient populations and community treatment providers. It also serves as a research and training platform to help NIDA respond to emerging public health areas. Currently, the CTN provides a research platform for 46 projects, including career development (K awards) for junior faculty. As the CTN celebrates its 10th anniversary, its value for increasing adoption of research-based treatments at the community level is undeniable. For example, CTN trials comparing buprenorphine/naloxone (marketed as Suboxone) to a non-opioid medication, previously the standard for detoxification, were so successful that they softened pervasive negative attitudes among treatment providers hesitant to use an opioid medication in people addicted to heroin. The trials demonstrated that Suboxone could be useful during the detoxification stage of treatment and provide a safe and effective maintenance treatment even for young people—garnering its greater acceptance and wider use by Community Treatment Programs.
Budget Policy: The FY 2012 budget estimate for this program is $45.643 million, an increase of $0.838 million and 1.8 percent over the FY 2010 estimate.
Intramural Research Program (IRP): This Intramural program performs cutting edge research within a coordinated multidisciplinary framework. The IRP attempts to elucidate the nature of the addictive process; to determine the potential use of new therapies for substance abuse, both pharmacological and psychosocial; and to decipher the long-term consequences of drugs of abuse on brain development, maturation, function, and structure, and on other organ systems. In addition, the IRP supports an HIV/AIDS Pathophysiology and Medications Discovery Program, which focuses on (1) how HIV or its products cross the blood-brain barrier, (2) how toxic compounds generated by HIV invade brain cells, and (3) the development of compounds to block the toxic effects of HIV on immune system cells. Recently published research findings provide critical new insights into how a genetic variant, previously associated with heightened risk for nicotine addiction and related illness, may actually contribute to nicotine addiction. Specifically, IRP researchers found evidence that carriers of a specific nicotinic receptor display different "resting state" patterns among key functionally connected regions of the brain's reward circuit. This observation is important because it begins to uncover meaningful differences in resting state functional connectivity, which can be revealed through use of functional Magnetic Resonance Imaging (fMRI) and may provide a new platform (or biomarker) for detecting disease vulnerabilities, including to drug abuse and addiction. Importantly, this finding heralds a new era for linking the accumulated body of research identifying specific genetic risk variants with neurobehavioral features likely to trigger or characterize addiction-related traits.
Budget Policy: The FY 2012 budget estimate for this program is $89.765 million, an increase of $2.200 million and 2.5 percent over the FY 2010 estimate.
Research Management and Support (RMS): RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. Additionally, the functions of RMS encompass strategic planning, coordination, and evaluation of NIDA's programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public. NIDA currently oversees more than 1,800 research grants and more than 190 research and development contracts. In addition to the infrastructure required to support research and training, NIDA also strives to educate the public about drug abuse and addiction and to raise awareness of the science behind it. This year, NIDA's launched its first ever "National Drug Facts Week"—a health awareness campaign directed at teens. Building on our popular Drug Facts Chat Day, in which students from across the country learn the facts about drug abuse from NIDA scientists interacting with them in cyberspace, National Drug Facts Week (November 2010) was staged in conjunction with dozens of "shatter the myths" events nationwide, that sought to debunk popular myths about drugs and instead give teens information they can use to make healthy decisions. The Department of Education was a key partner in developing and disseminating this initiative. In addition, the interactive Sara Bellum Blog (SBB), a key feature of NIDA's innovative teen website, regularly shared science-based messages about drug use and health, allowing and responding to comments from thousands of teen readers. In FY 2010, the blog received several awards for its straightforward style and overall appeal.
Budget Policy: The FY 2012 budget estimate for this program is $62.247 million, an increase of $1.527 million and 2.5 percent over the FY 2010 estimate.
NIH Collaborative Activities: NIDA participates in a variety of activities supported through the Common Fund; the Basic Behavioral and Social Sciences Opportunity Network, or OppNet; and the Neuroscience Blueprint. Among these, NIDA has a lead role on (1) the Epigenomics-funded grants initiated under Roadmap; (2) an OppNet-supported RFA (DA-11-003) titled the Effects of the Social Environment on Health, which will fund research to investigate structural, behavioral, sociocultural, environmental, cognitive, emotional, and/or biological mechanisms by which the social environment affects health outcomes; and (3) the NIH Blueprint-supported Institutional Training Grants on Computational Neuroscience and Neuroimaging—Integrating First Principles and Applications.
References
- http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm229109.htm
- Hatsukami et al., Pharmacodynamics and Drug Action, 2005; Kosten, Archives of General Psychiatry, 2009.
- Ibid.
- http://www.nabi.com/pipeline/clinicaltrials.php
- DeCharms, RC. Applications of real-time fMRI. Nat Rev Neurosci. 9(9):720-729, 2008. AND DeCharms et al., Control over brain activation and pain learned by using real-time functional MRI. PNAS 102(51):18626Ð18631, 2006.
- Miniussi C, Cappa SF, Cohen LG, Floel A, Fregni F, Nitsche MA, Oliveri M, Pascual-Leone A, Paulus W, Priori A, Walsh V. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul. 2008 Oct;1(4):326-36.
- https://www.cdc.gov/hiv/statistics/overview/ataglance.html
- http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf (PDF, 3.1MB)
- https://obamawhitehouse.archives.gov/ondcp/ondcp-fact-sheets
- https://www.samhsa.gov/data/all-reports?f%5B0%5D=survey_type%3A377
- Krupitsky E, American Psychiatric Association Annual Meeting, May 26, 2010, New Orleans, LA.