Organizational Chart
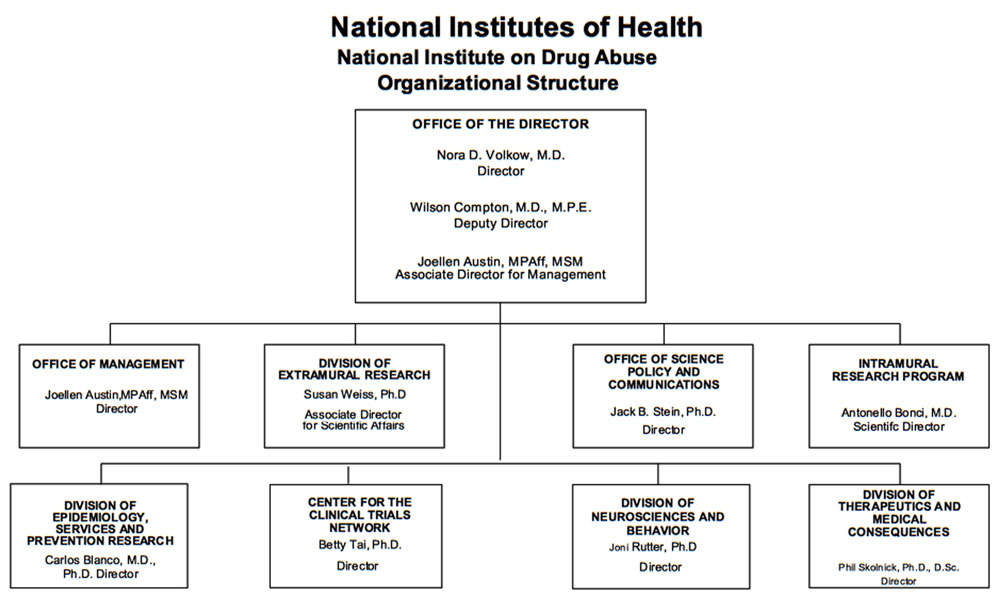
- Description of Organizational Chart
-
Office of the Director
Nora D. Volkow, M.D.
DirectorWilson Compton, M.D., M.P.E.
Deputy DirectorJoellen Austin, MPAff, MSM
Associate Director for ManagementOffice of Management
Joellen Austin, MPAff, MSM
DirectorDivision of Extramural Research
Susan Weiss, Ph.D.
Associate Director for Scientific AffairsOffice of Science Policy and Communications
Jack B. Stein, Ph.D.
DirectorIntramural Research Program
Antonello Bonci, M.D.
Scientific DirectorDivision of Epidemiology, Services and Prevention Research
Carlos Blanco, M.D., Ph.D.
DirectorCenter for the Clinical Trials Network
Betty Tai, Ph.D.
DirectorDivision of Neurosciences and Behavioral Research
Joni Rutter, Ph.D.
DirectorDivision of Therapeutics and Medical Consequences
Phil Skolnick, Ph.D., D.Sc.
Director
Appropriation Language
For carrying out section 301 and title IV of the PHS Act with respect to drug abuse, [$1,077,488,000] $1,020,459,000.
Tables
- Amounts Available for Obligation Table
-
Amounts Available for Obligation 1
(Dollars in Thousands)Source of Funding FY 2015
ActualFY 2016
EnactedFY 2017
President's
BudgetAppropriation $1,028,614 $1,077,488 $1,050,550 Mandatory Appropriation: (non-add)
Type 1 Diabetes(0) (0) (0) Mandatory Appropriation: (non-add)
Other Mandatory financing(0) (0) (30,091) Rescission 0 0 0 Sequestration 0 0 0 FY 2015 First Secretary's Transfer 0 0 0 FY 2015 Second Secretary's Transfer 0 0 0 Subtotal, adjusted appropriation $1,028,614 $1,077,488 $1,050,550 OAR HIV/AIDS Transfers -12,909 -26,938 0 National Children's Study Transfers 0 0 0 Subtotal, adjusted budget authority $1,015,705 $1,050,550 $1,050,550 Unobligated balance, start of year 0 0 0 Unobligated balance, end of year 0 0 0 Subtotal, adjusted budget authority $1,015,705 $1,050,550 $1,050,550 Unobligated balance lapsing -10 0 0 Total obligations $1,015,695 $1,050,550 $1,050,550 1 Excludes the following amounts for reimbursable activities carried out by this account:
FY 2015 - $72,664 FY 2016 - $44,516 FY 2017 - $101,502
- Budget Mechanism Table
-
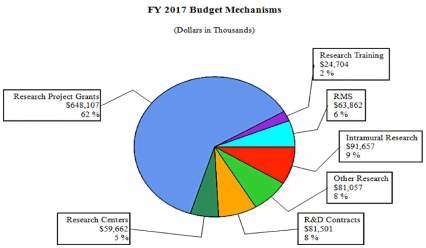
Budget Mechanism - Total1 (Dollars in Thousands) Mechanism FY 2015
ActualFY 2016
EnactedFY 2017
President's
Budget3FY 2017
+/-
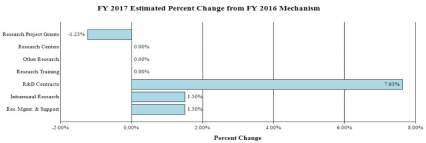
FY 2016No. Amount No. Amount No. Amount No. Amount Research Projects: Noncompeting 887 $426,545 942 $484,021 878 $462,268 -64 -$21,753 Administrative Supplements (115) 6,968 (116) 7,000 (116) 7,000 (0) 0 Competing: Renewal 46 27,824 47 28,500 53 42,485 6 13,985 New 313 142,568 269 111,884 265 109,917 -4 -1,967 Supplements 0 0 0 0 0 0 0 0 Subtotal, Competing 359 $170,392 316 $140,384 318 $152,402 2 $12,018 Subtotal, RPGs 1,246 $603,905 1,258 $631,405 1,196 $621,670 -62 -$9,735 SBIR/STTR 46 22,173 46 24,778 49 26,437 3 1,659 Research Project Grants 1,292 $626,078 1,304 $656,183 1,245 $648,107 -59 -$8,076 Research Centers: Specialized / Comprehensive 34 $58,491 34 $59,662 34 $59,662 0 0 Clinical Research 0 0 0 0 0 0 0 0 Biotechnology 0 0 0 0 0 0 0 0 Comparative Medicine 0 0 0 0 0 0 0 0 Research Centers in Minority Institutions 0 0 0 0 0 0 0 0 Research Centers 34 $58,491 34 $59,662 34 $59,662 0 0 Other Research: Research Careers 200 $34,080 200 $34,421 200 $34,421 0 0 Cancer Education 0 0 0 0 0 0 0 0 Cooperative Clinical Research 13 30,399 13 30,399 13 30,399 0 0 Biomedical Research Support 0 0 0 0 0 0 0 0 Minority Biomedical Research Support 0 1,311 0 1,311 0 1,311 0 0 Other 70 14,926 71 14,926 71 14,926 0 0 Other Research 283 $80,716 284 $81,057 284 $81,057 0 0 Total Research Grants 1,609 $765,285 1,622 $796,902 1,563 $788,826 -59 -$8,076 Ruth L. Kirschstein Training Awards FTTPs FTTPs FTTPs FTTPs Individual Awards 135 $5,430 136 $5,634 135 $5,634 -1 0 Institutional Awards 411 18,301 417 19,070 415 19,070 -2 0 Total Research Training 546 $23,731 553 $24,704 550 $24,704 -3 0 Research and Development Contracts 87 $75,723 87 $75,723 87 $81,501 0 $5,778 (SBIR/STTR) (non-add)2 (15) (6,442) (14) (6,200) (14) (6,200) 0 0 Intramural Research 128 $88,978 129 $90,303 129 $91,657 0 $1,354 Res. Management & Support 268 61,988 271 62,918 271 63,862 0 944 Res. Management & Support (SBIR Admin) (non-add)2 0 (340) 0 (360) 0 0 0 (-360) Office of the Director - Appropriation2 0 0 0 0 0 0 0 0 Office of the Director - Other 0 0 0 0 0 0 0 0 ORIP/SEPA (non-add)2 0 0 0 0 0 0 0 0 Common Fund (non-add)2 0 0 0 0 0 0 0 0 Buildings and Facilities 0 0 0 0 0 0 0 0 Appropriation 0 0 0 0 0 0 0 0 Type 1 Diabetes 0 0 0 0 0 0 0 0 Program Evaluation Financing 0 0 0 0 0 0 0 0 Cancer Initiative Mandatory Financing 0 0 0 0 0 0 0 0 Other Mandatory Financing 0 0 0 0 0 -30,091 0 -30,091 Subtotal, Labor/HHS Budget Authority 0 $1,015,705 0 $1,050,550 0 $1,020,459 0 -$30,091 Interior Appropriation for Superfund Res. 0 0 0 0 0 0 0 0 Total, NIH Discretionary B.A. 0 $1,015,705 0 $1,050,550 0 $1,020,459 0 -$30,091 Type 1 Diabetes 0 0 0 0 0 0 0 0 Proposed Law Funding 0 0 0 0 0 0 0 0 Cancer Initiative Mandatory Financing 0 0 0 0 0 0 0 0 Other Mandatory Financing 0 0 0 0 0 30,091 0 30,091 Total, NIH Budget Authority 0 $1,015,705 0 $1,050,550 0 $1,050,550 0 0 Program Evaluation Financing 0 0 0 0 0 0 0 0 Total, Program Level 0 $1,015,705 0 $1,050,550 0 $1,050,550 0 0 1 All Subtotal and Total numbers may not add due to rounding.
2 All numbers in italics and brackets are non-add.
3 Includes mandatory financing.
- Summary of Changes Table
-
Summary of Changes
(Dollars in Thousands)FY 2016 Enacted $1,050,550 FY 2017 President's Budget $1,050,550 Net change $0 CHANGES 2017 President's Budget1: FTEs 2017 President's Budget1: Budget Authority Change from FY 2016: FTEs Change from FY 2016: Budget Authority A. Built-in: 1. Intramural Research: a. Annualization of January 2016 pay increase & benefits $25,165 $86 b. January FY 2017 pay increase & benefits 25,165 259 c. Two less days of pay 25,165 -95 d. Differences attributable to change in FTE 25,165 0 e. Payment for centrally furnished services 10,188 248 f. Increased cost of laboratory supplies, materials, other expenses, and non-recurring costs 56,304 854 Subtotal $1,354 2. Research Management and Support: a. Annualization of January 2016 pay increase & benefits $36,035 $127 b. January FY 2017 pay increase & benefits 36,035 382 c. Two less days of pay 36,035 -136 d. Differences attributable to change in FTE 36,035 0 e. Payment for centrally furnished services 5,694 139 f. Increased cost of laboratory supplies, materials, other expenses, and non-recurring costs 22,133 432 Subtotal $944 Subtotal, Built-in $2,298 Summary of Changes - Continued
(Dollars in Thousands)CHANGES Fy 2017 President's Budget1: No. Fy 2017 President's Budget1: Amount Change from
FY 2016 No.Change from
FY 2016: AmountB. Program: 1. Research Project Grants: a. Noncompeting 878 $469,268 -64 -$21,753 b. Competing 318 152,402 2 12,018 c. SBIR/STTR 49 26,437 3 1,659 Subtotal, RPGs 1,245 $648,107 -59 -$8,076 2. Research Centers 34 $59,662 0 $0 3. Other Research 284 81,057 0 0 4. Research Training 550 24,704 -3 0 5. Research and development contracts 87 81,501 0 5,778 Subtotal, Extramural $895,031 -$2,298 FTEs FTEs 6. Intramural Research 129 $91,657 0 -$0 7. Research Management and Support 271 63,862 0 0 8. Construction 0 0 9. Buildings and Facilities 0 0 Subtotal, program 400 $1,050,550 0 -$2,298 Total changes $0 1 Includes mandatory financing.
-
Budget Authority by Activity1
(Dollars in thousands)Extramural Research FY 2015
ActualFY 2016
EnactedFY 2017
President's
Budget2FY 2017
+/-
FY 2016FTE Amount FTE Amount FTE Amount FTE Amount Detail: Division of Therapeutics and Medical Consequences $178,838 $178,380 -$458 Division of Neuroscience and Behavior 351,035 350,136 -899 Division of Basic Neuroscience & Behavioral Research 429,869 0 Division of Epidemiology, Services and Prevention Research 265,357 324,205 323,375 -830 Pharmacotherapies and Medical Consequences 127,795 0 0 0 Center for the Clinical Trials Network 41,718 43,251 43,140 -111 Subtotal, Extramural $864,739 $897,329 $895,031 -$2,298 Intramural Research 128 $88,978 129 $90,303 129 $91,657 0 $1,354 Research Management & Support 268 $61,988 271 $62,918 271 $63,862 0 $944 TOTAL 396 $1,015,705 400 $1,050,550 400 $1,050,550 0 $0 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
2Includes mandatory financing.
-
Authorizing Legistlation PHS Act/Other Citation U.S. Code Citation 2016 Amount Authorized FY 2016 Enacted 2017 Amount Authorized FY 2017 President's Budget1 Research and Investigation Section 301 42§241 Indefinite $1,050,550,000 Indefinite $1,020,459,000 National Institute on Drug Abuse Section 401(a) 42§281 Indefinite Indefinite Total, Budget Authority $1,050,550,000 $1,020,459,000 1 Excludes mandatory financing.
- Appropriations History Table
-
Appropriations History Fiscal Year Budget Estimate to Congress House Allowance Senate Allowance Appropriation 2007 $994,829,000 $994,829,000 $1,000,342,000 $1,000,621,000 Rescission $0 2008 $1,000,365,000 $1,015,559,000 $1,022,594,000 $1,018,493,000 Rescission $17,793,000 Supplemental $5,322,000 2009 $1,001,672,000 $1,035,997,000 $1,029,539,000 $1,032,759,000 Rescission $0 2010 $1,045,384,000 $1,069,583,000 $1,050,091,000 $1,059,848,000 Rescission $0 2011 $1,094,078,000 $1,092,369,000 $1,059,848,000 Rescission $9,306,097 2012 $1,080,018,000 $1,080,018,000 $1,038,714,000 $1,055,362,000 Rescission $1,994,634 2013 $1,054,001,000 1,057,196,000 $1,053,367,366 Rescission $2,106,735 Sequestration ($52,871,798) 2014 $1,071,612,000 $1,064,490,000 $1,025,435,000 Rescission $0 2015 $1,023,268,000 $1,028,614,000 Rescission $0 2016 $1,047,397,000 $1,050,875,000 $1,069,086,000 $1,077,488,000 Rescission $0 20171 $1,050,550,000 1 Includes mandatory financing.
-
Budget Authority by Object Class1
(Dollars in Thousands)FY 2016 Enacted FY 2017 President's Budget2 Fy 2017 +/- Fy 2016 Total compensable workyears: Full-time employment 400 400 0 Full-time equivalent of overtime and holiday hours 0 0 0 Average ES salary $183 $186 $2 Average GM/GS grade 12.6 12.6 0.0 Average GM/GS salary $108 $110 $1 Average salary, grade established by act of July 1, 1944 (42 U.S.C. 207) $109 $110 $1 Average salary of ungraded positions $145 $147 $2 OBJECT CLASSES FY 2016 Enacted FY 2017 President's Budget2 Fy 2017 +/- Fy 2016 Personnel Compensation: 11.1 Full-time permanent $29,154 $29,376 $222 11.3 Other than full-time permanent 10,913 10,996 83 11.5 Other personnel compensation 1,008 1,016 8 11.7 Military personnel 862 868 7 11.8 Special personnel services payments 4,749 4,785 36 11.9 Subtotal Personnel Compensation $46,686 $47,042 $356 12.1 Civilian personnel benefits $13,390 $13,656 $265 12.2 Military personnel benefits 499 502 4 13.0 Benefits to former personnel 0 0 0 Subtotal Pay Costs $60,575 $61,200 $625 21.0 Travel and transportation of persons $861 $861 $0 22.0 Transportation of things 131 131 0 23.1 Rental payments to GSA 0 0 0 23.2 Rental payments to others 0 0 0 23.3 Communications, utilities and miscellaneous charges 432 432 0 24.0 Printing and reproduction 1 1 0 25.1 Consulting services $1,052 $1,052 $0 25.2 Other services 6,149 6,149 0 25.3 Purchase of goods and services from government accounts 111,756 119,300 7,544 25.4 Operation and maintenance of facilities $1,704 $1,704 $0 25.5 Research and development contracts 38,574 38,482 -93 25.6 Medical care 307 307 0 25.7 Operation and maintenance of equipment 1,903 1,903 0 25.8 Subsistence and support of persons 0 0 0 25.0 Subtotal Other Contractual Services $161,445 $168,897 $7,451 26.0 Supplies and materials $5,010 $5,010 $0 31.0 Equipment 5,256 5,256 0 32.0 Land and structures 0 0 0 33.0 Investments and loans 0 0 0 41.0 Grants, subsidies and contributions 816,837 808,761 -8,076 42.0 Insurance claims and indemnities 0 0 0 43.0 Interest and dividends 2 2 0 44.0 Refunds 0 0 0 Subtotal Non-Pay Costs $989,975 $989,350 -$625 Total Budget Authority by Object Class $1,050,550 $1,050,550 $0 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
2Includes mandatory financing.
- Salaries and Expenses Table
-
Salaries and Expenses
(Dollars in Thousands)OBJECT CLASSES FY 2016 Enacted FY 2017 President's Budget FY 2017 +/- FY 2016 Personnel Compensation: Full-time permanent (11.1) $29,154 $29,376 $222 Other than full-time permanent (11.3) 10,913 10,996 83 Other personnel compensation (11.5) 1,008 1,016 8 Military personnel (11.7) 862 868 7 Special personnel services payments (11.8) 4,749 4,785 36 Subtotal Personnel Compensation (11.9) $46,686 $47,042 $356 Civilian personnel benefits (12.1) $13,390 $13,656 $265 Military personnel benefits (12.2) 499 502 4 Benefits to former personnel (13.0) 0 0 0 Subtotal Pay Costs $60,575 $61,200 $625 Travel and transportation of persons (21.0) $861 $861 $0 Transportation of things (22.0) 131 131 0 Rental payments to others (23.2) 0 0 0 Communications, utilities and miscellaneous charges (23.3) 432 432 0 Printing and reproduction (24.0) 1 1 0 Other Contractual Services: Consultant services (25.1) 923 923 0 Other services (25.2) 6,149 6,149 0 Purchases from government accounts (25.3) 74,294 76,060 1,766 Operation and maintenance of facilities (25.4) 1,704 1,704 0 Operation and maintenance of equipment (25.7) 1,903 1,903 0 Subsistence and support of persons (25.8) 0 0 0 Subtotal Other Contractual Services $84,972 $86,739 $1,766 Supplies and materials (26.0) $5,010 $5,010 $0 Subtotal Non-Pay Costs $91,408 $93,174 $1,766 Total Administrative Costs $151,983 $154,374 $2,391
- Detail of Full-Time Equivalent Employment (FTE) Table
-
Details of Full-Time Equivalent Employment (FTE) OFFICE/DIVISION FY 2015 Actual FY 2016 Est. FY 2017 Est. Civilian Military Total Civilian Military Total Civilian Military Total Center for the Clinical Trials Network Direct: 13 1 14 12 1 13 12 1 13 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 13 1 14 12 1 13 12 1 13 Division of Basic Neuroscience & Behavioral Research Direct: 24 0 24 0 0 0 0 0 0 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 24 0 24 0 0 0 0 0 0 Division of Clinical Neuroscience and Behavioral Research Direct: 15 0 15 0 0 0 0 0 0 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 15 0 15 0 0 0 0 0 0 Division of Epidemiology, Services and Prevention Research Direct: 24 1 25 29 1 30 29 1 30 Reimbursable: 2 0 2 2 0 2 2 0 2 Total: 26 1 27 31 1 32 31 1 32 Division of Extramural Research Direct: 34 0 34 38 0 38 38 0 38 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 34 0 34 38 0 38 38 0 38 Division of Neuroscience and Behavior Direct: 0 0 0 30 0 30 30 0 30 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 0 0 0 30 0 30 30 0 30 Division of Pharmacotherapies and Medical Consequences of Drug Abuse Direct: 31 0 31 0 0 0 0 0 0 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 31 0 31 0 0 0 0 0 0 Division of Therapeutics and Medical Consequences Direct: 0 0 0 33 0 33 33 0 33 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 0 0 0 33 0 33 33 0 33 Intramural Research Program Direct: 125 0 125 126 0 126 126 0 126 Reimbursable: 3 0 3 3 0 3 3 0 3 Total: 128 0 128 129 0 129 129 0 129 Office of Management Direct: 41 0 41 41 0 41 41 0 41 Reimbursable: 36 0 36 36 0 36 36 0 36 Total: 77 0 77 77 0 77 77 0 77 Office of Science Policy and Communication Direct: 27 0 27 27 0 27 27 0 27 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 27 0 27 27 0 27 27 0 27 Office of the Director Direct: 19 0 19 21 0 21 21 0 21 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 19 0 19 21 0 21 21 0 21 Total (Includes FTEs whose payroll obligations are supported by the NIH Common Fund) 394 2 396 398 2 400 398 2 400 FTEs supported by funds from Cooperative Research and Development Agreements 0 0 0 0 0 0 0 0 0 Fiscal Year Average GS Grade 2013 12.8 2014 12.7 2015 12.5 2016 12.6 2017 12.6
- Detail of Positions Table
-
Detail of Positions1 GRADE FY 2015 Actual FY 2016 Enacted FY 2017 President's Budget Total, ES Positions 1 1 1 Total, ES Salary 181,500 183,300 185,500 GM/GS-15 152,806 154,640 156,495 GM/GS-14 132,007 133,591 135,194 GM/GS-13 106,315 107,591 108,882 GS-12 90,973 92,065 93,169 GS-11 70,246 71,089 71,942 GS-10 0 0 0 GS-9 58,408 59,109 59,818 GS-8 59,013 59,721 60,438 GS-7 49,155 49,745 50,342 GS-6 48,436 49,017 49,605 GS-5 38,236 38,695 39,159 GS-4 31,069 31,442 31,819 GS-3 27,675 28,007 28,343 GS-2 0 0 0 GS-1 0 0 0 Subtotal 864,339 874,712 885,206 Grades established by Act of July 1, 1944 (42 U.S.C. 207): 0 0 0 Assistant Surgeon General 0 0 0 Director Grade 120,221 121,664 123,124 Senior Grade 96,636 97,796 98,969 Full Grade 0 0 0 Senior Assistant Grade 0 0 0 Assistant Grade 0 0 0 Subtotal 216,857 219,460 222,093 Ungraded 143,607 145,330 147,074 Total permanent positions 317 321 321 Total positions, end of year 409 413 413 Total full-time equivalent (FTE) employment, end of year 396 400 400 Average ES salary 181,500 183,300 185,500 Average GM/GS grade 12.5 12.6 12.6 Average GM/GS salary 108,455 108,384 109,685 1Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
Major Changes in the Fiscal Year 2017 President’s Budget Request
The FY 2017 President’s Budget for NIDA is $1,050.550 million, which is the same as the FY 2016 Enacted level, results in no major changes.
Budget Graphs
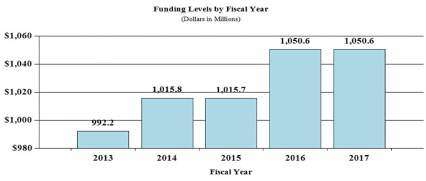
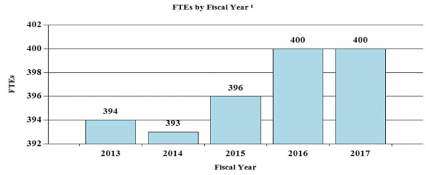
History of Budget Authority and FTEs:
Distribution by Mechanism (dollars in thousands):
Change by Selected Mechanism:
Justification of Budget Request
National Institute on Drug Abuse
Authorizing Legislation: Section 301 and Title IV of the Public Health Service Act, as amended.
| FY 2015 Actual | FY 2016 Enacted | FY 2017 President's Budget | FY 2017 +/- FY 2016 | |
|---|---|---|---|---|
| BA | $1,015,695,285 | $1,050,550,000 | $1,050,550,000 | 0 |
| FTE | 396 | 400 | 400 | 0 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Director's Overview
Substance use and its consequences take an enormous toll on our Nation. The combined healthcare, crime-related, and productivity costs alone exceed $600 billion a year, but dollars poorly approximate the devastating human cost of substance use disorders (SUDs). Drug abuse and addiction present complex and continuously evolving challenges currently dominated by an ongoing epidemic of prescription opioid abuse that is now contributing to a rise in heroin use as well as dramatic HCV and HIV outbreaks; by the emergence of new synthetic drugs as well as new drug delivery systems such as e-cigarettes and vaporizers that are changing how people use drugs; and by rapidly changing State policies and social norms related to marijuana. The National Institute on Drug Abuse (NIDA) strives to meet these challenges by strategically supporting and conducting basic, clinical, and services research on drug use, its consequences, and the underlying neurobiological and behavioral mechanisms involved, and by working with Federal, State, and community partners to support the effective translation and implementation of scientific research findings to improve the prevention and treatment of SUDs.
Basic research: Understanding the neurobiology of addiction
Drug use and addiction are mediated by numerous biological, environmental, social, behavioral, and developmental factors. A more detailed understanding of the interactions among these factors and how they contribute to the risk for addiction and other negative consequences of drug use is critical for developing better prevention and treatment strategies. NIDA supports a robust portfolio of basic research to understand the mechanisms that underlie the transition from drug use to addiction and the brain changes that characterize SUDs. One illustrative example of this type of research is the Adolescent Brain Cognitive Development (ABCD) study, a landmark effort led by NIDA in partnership with the National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Cancer Institute (NCI), and other NIH partners (the Collaborative Research on Addiction at NIH, or CRAN) that officially launched in September 2015 with the award of 13 grants. Researchers will recruit approximately 10,000 children at age 9 or 10 – before initiation of drug use – and collect detailed neuroimaging, genetic, behavioral, environmental and other health data at periodic intervals over the course of a decade. Data produced by this study will enable scientists to assess the effects of different patterns of substance use (including no use) on the developing brain, mental health, and outcomes like academic achievement, as well as a wide range of other factors affecting young Americans’ lives and health.
Leveraging Technology Advances: Big Data and Precision Medicine
The rapid progress of addiction research in the past few years has been fueled in part by technological advances, particularly in brain imaging, high-throughput genotyping, genetic tools for the manipulation of neuronal circuits, and health information technology (HIT) tools (e.g., electronic health records and mobile health applications). Addiction science is fast approaching a data analysis bottleneck. For example, sequencing technologies have reached the point where it is now far cheaper to sequence whole genomes than to analyze the results. NIDA has begun a long-term effort – known as the Addictome Project – to develop the infrastructure necessary to analyze complex systems (drawing from mathematics, statistics, engineering, computer science, and bioinformatics) in ways that allow researchers to investigate behaviors of non-linear, highly interactive systems. Such analytical and modeling tools are urgently required to take full advantage of the emerging data sets and to address multi-faceted questions, such as how genes linked to addiction influence brain function and the response to drugs of abuse; and how social and environmental stimuli can interact to influence brain circuit function. Understanding the complexity of interacting biological, environmental, and social factors that contribute to and sustain SUDs also presents significant opportunities for the application of precision medicine. An individual’s environment, experience, and biology combine to determine their risk for developing an SUD, the trajectory the SUD will take, and the interventions that will be most effective for treating it. This is the inspiration behind the President’s Precision Medicine Initiative, which will provide a foundation for the development of personalized interventions addressing the multiplicity of individual characteristics.
Strategic Partnerships: Advancing Discovery and Translation
SUDs represent complex public health problems that require coordinated, multi-faceted efforts to address. NIDA engages in numerous strategic partnerships to achieve its mission. For example, the ongoing epidemic of prescription opioid and heroin abuse is a serious national issue with devastating consequences, including not just opioid use disorders and related overdoses, but also a rising incidence of newborns who experience neonatal abstinence syndrome and increased spread of infectious diseases, including HIV and hepatitis C. Existing effective treatments are highly underutilized across the United States. As part of the Department of Health and Human Services’ Opioid Initiative, NIDA is partnering with other Federal agencies to address this crisis.
The Opioid Initiative emphasizes the implementation of evidence-based prevention and treatment strategies, which include not only better prescription practices but also deployment of medication to combat overdoses (such as naloxone) and medication – assisted treatment (MAT) to treat opioid use disorders. Under this initiative, NIDA is focused on supporting research and disseminating research findings to: 1) improve opioid prescribing practices; 2) expand the use of naloxone; 3) improve the integration of MAT pharmacotherapies into treatment services in specialty care and primary care; and 4) develop pain treatments with reduced potential for misuse and diversion.
To achieve these goals, NIDA is working with diverse community stakeholders. For example, NIDA has initiated multiple strategic partnerships to advance development of medications for pain, leveraging NIDA funds with the strengths and resources of outside organizations, including academic institutions, pharmaceutical and biotechnology companies, private and public foundations, and small businesses. This includes research to identify new pain medicines with reduced abuse, tolerance, and dependence risk; developing new formulations of pain and addiction treatment medications that minimize diversion and non-medical use (e.g., by preventing tampering) and reduce the risk of overdose deaths. For example, NIDA has partnered with Titan Pharmaceuticals and Braeburn Pharmaceuticals to develop a formulation of buprenorphine to be implanted under the skin. This formulation, called Probuphine, would deliver a steady dose of buprenorphine for six months, an innovation that could improve patient adherence to treatment for opioid use disorder (see program portrait).
NIDA is also working closely with community stakeholders to improve education of healthcare providers on both pain management and addressing SUDs. For example, NIDA, in partnership with the NIH Pain Consortium, funds 11 Centers of Excellence for Pain Education (CoEPEs) that act as hubs for the development, evaluation, and distribution of pain-management curriculum resources for medical, dental, nursing, and pharmacy schools that address SUD risk.
Program Descriptions and Accomplishments
Basic and Clinical Neuroscience: The Basic and Clinical Neuroscience portfolio seeks to expand our understanding of the fundamental neurological, genetic/epigenetic, and behavioral processes that underlie SUDs. Central to this goal are efforts to tease apart the multiple factors that contribute to drug abuse and addiction risk, with particular attention to individual differences in risk and responses to drugs while at the same time expanding our basic knowledge of the function of the brain from the molecular to the behavioral level. Key projects are investigating the effects of drugs on gene expression and brain development and function, and exploring gender-related differences in these effects. Risk for addiction is profoundly affected by an individual’s genes as well as environmental conditions, such as stress and early exposure to drugs of abuse. Additional studies are exploring the mechanisms underlying these effects, including the role of epigenetic changes that can influence long-term patterns of gene expression in specific brain cells (neuron and glia) without changing DNA sequence. NIDA is leading the NIH Common Fund (CF) Epigenomics Program and co-leads a CF workgroup to eukaryotic epitranscriptomics. A relatively new focus is to develop the capacity to support big data science to promote efficient analysis of large integrated data sets on a scale not previously possible (see program portrait). NIDA is the lead for the NIH Blueprint-supported Neuroscience Information Framework, a dynamic inventory of Web-based neuroscience resources, data, and tools for scientists and students. Collectively, these efforts will improve our understanding of the basic neural and genetic mechanisms that underlie drug abuse and addiction and will provide critical insights toward the development of more effective approaches for the prevention and treatment of SUDs. For example, continuing efforts to improve our understanding of the endocannabinoid system are opening up new areas of investigation for the development of novel pain and addiction treatments. Other projects are exploring the basic processes underlying resilience against SUDs in childhood and adolescence. In addition, and in line with the goals of the President’s Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative, NIDA is supporting research to: 1) develop advanced technologies that improve our ability to study the organization and function of the living brain; 2) better understand the interactions of complex neural circuits including those that mediate reward, aversion to drug effects, and related decision making; and 3) develop novel strategies to therapeutically influence SUD-relevant brain circuits including transcranial magnetic and deep brain stimulation, and neurofeedback. Progress in these combined areas will revolutionize our ability to mitigate or even reverse the deleterious effects of SUDs.
Budget Policy: The FY 2017 Budget estimate is $350.136 million, which is $0.899 million or 0.3 percent less than the FY 2016 Enacted level.
Program Portrait: Big Data for Addiction Science
FY 2016 Level: $1.4 million
FY 2017 Level: $1.4 million
Change: $0.0 million
The rapid progress of the past few years of addiction research has been fueled in part by technological advances, particularly in next-generation sequencing, genomics, epigenetics, epidemiology and neuroimaging. These advances have enhanced our understanding of the biological, developmental, and environmental factors that influence brain function in health and disease. And now ‘Big Data’ is providing unprecedented new opportunities to maximize the value of data, giving researchers the ability to analyze large amounts of data in new ways – turning vast datasets of complex information into knowledge.
Big Data is more than just large data sets; it refers to the complexity, challenges, and opportunities presented by combining and analyzing different types of data in an integrated way. In SUD research, these data sources include imaging, genetic, molecular, exposure, health, behavioral, and many other types of data being generated by researchers, hospitals, and mobile devices around the world. These data have the power to speed discovery of new treatments, to determine the genetic and environmental causes of SUDs, to support the development of precision medicine in healthcare, and so much more. There are certainly challenges to overcome – from how and where to store massive data sets; how to integrate and harmonize data; how to ensure data quality and consistency; how to facilitate efficient use of available data; how to foster a culture of open science and data sharing; how to ensure privacy and confidentiality; among others – but NIDA and the NIH Big Data to Knowledge (BD2K) program are committed to addressing these challenges and helping the biomedical research community harness the potential of Big Data.
NIDA’s Addictome Project will work to develop a research database that integrates diverse multi-scale data sets that can be mined by the scientific community and visualized in a user-friendly framework to support discovery of novel relationships and scientific knowledge related to addiction. The Addictome will be a collection of numerous data types from diverse sources representing factors that contribute to an individual’s risk for addiction across the lifespan. It will provide the infrastructure and tools necessary to enable investigation into how these diverse factors interact within and across individuals to influence addiction risk and lead to diverse substance use trajectories.
Epidemiology, Services, and Prevention Research: This portfolio supports integrated approaches to understand and develop strategies to address the interactions between individuals and environments that contribute to drug abuse and related problems. With a focus on research to inform public health, the program includes large surveys – such as the annual Monitoring the Future survey, which tracks drug use and related attitudes among teens – and surveillance networks to monitor issues and trends locally and nationally. NIDA’s National Drug Early Warning System (NDEWS) monitors emerging trends related to illicit drug use including designer synthetic compounds and heroin. NDEWS generates critical information about new drug trends in specific locations around the country so that rapid, informed, and effective public health responses can be developed and implemented precisely where and when they are needed. NIDA also supports research related to treatment of SUDs in the criminal justice system, including studies that pertain to the implementation of MAT and seek, test, treat, and retain (STTR) strategies for people with SUDs at risk for HIV. Program efforts also guide development of preventive interventions for a variety of populations; as well as research to optimize implementation and service delivery in real-world settings. The program also includes research to better understand the impact of policy changes related to substance use, including implementation of health reform and parity regulations and changes in State policies related to marijuana. Specifically, current research is examining the impact of health reform on access to quality treatment for persons with SUDs, as well as associations between changes in State marijuana policies and trends in use, harm perception, health consequences including trauma and car crashes, and educational outcomes, particularly for adolescents and young adults. Such knowledge can be then used to inform policy and to improve prevention and treatment interventions.
Budget Policy: The FY 2017 Budget estimate is $323.375 million, which is $0.830 million or 0.3 percent less than the FY 2016 Enacted level.
Program Portrait: Low-nicotine Cigarettes
FY 2016 Level: $0.6 million
FY 2017 Level: $0.6 million
Change: $0.0 million
Smoking is the leading preventable cause of disease, disability, and death in the United States. Over 480,000 Americans die each year from smoking and second hand smoke exposure – more than from homicides, AIDS, suicide, and car crashes, combined.1 In 2014, an estimated 65.8 million people, including over three million adolescents, smoked cigarettes.2 To help address this problem, the Tobacco Control Act, signed in 2009, authorized FDA to establish the Center for Tobacco Products to ensure that evidence-based science informs related regulatory actions. In 2013, NIDA and other NIH ICs partnered with FDA to support the development and evaluation of tobacco product regulations through its Tobacco Regulatory Science Program (TRSP).
Reduction in nicotine content has been proposed as a potential regulatory measure to render cigarettes non-addictive and, consequently, to reduce smoke exposure and improve public health. Cigarettes with low nicotine content have been associated with positive outcomes such as reduced smoking and reduced nicotine exposure and dependence. Several studies supported through the NIDA TRSP initiative are building on this research to evaluate how low nicotine cigarettes affect exposure and addiction and how low dose nicotine cigarettes affect vulnerable populations. One study evaluated the effects of smoking cigarettes that contained different levels of nicotine among current smokers. The average number of cigarettes smoked per day was lower after six weeks in participants randomly assigned to cigarettes containing lower amounts of nicotine. Compared with the normal (control) cigarettes, the cigarettes with lower levels of nicotine decreased exposure to and dependence on nicotine and also craving during abstinence from smoking.
Another study supported through the TRSP initiative is examining how low nicotine cigarettes affect smoking behavior in vulnerable populations. These include women of childbearing age or pregnant women, individuals with comorbid substance use disorders, and individuals with comorbid serious mental illness. Each of these populations is at increased risk for tobacco use and addiction or tobacco related adverse health outcomes. Yet despite these serious vulnerabilities, these populations have typically been excluded from tobacco regulatory studies.
Therapeutics and Medical Consequences: Since the pharmaceutical industry has traditionally made limited investment in the development of medications for SUDs, the responsibility for their development has rested largely with NIDA; NIDA, therefore, has developed a program to develop therapeutics for the treatment of SUDs. To most effectively leverage NIDA resources, this program encourages the formation of alliances between strategic partners (pharmaceutical and biotechnology companies as well as academic institutions) with the common goal of advancing medications through the development pipeline toward FDA approval in a timely manner. NIDA supports research to decrease the risks associated with medications development to make it more appealing for pharmaceutical companies to complete costly Phase IIb and III clinical studies. An example of such a project is a partnership with AstraZeneca to explore a novel medication that modulates the activity of glutamate – an excitatory neurotransmitter – to treat drug addiction. Preclinical studies with this class of molecule indicate that it could be effective for treating abuse of various drugs such as tobacco and cocaine. Another example is a partnership with two biotechnology companies to support the development of an intranasal formulation of the opioid overdose reversal drug naloxone. In addition, NIDA collaborated with Teva Pharmaceutical Industries on a clinical trial to test the efficacy and safety of a cholinesterase compound that has shown promise in pre-clinical trials for the treatment of cocaine addiction. NIDA continues to actively seek potential partners in the pharmaceutical and biotechnology sectors to develop novel therapeutics for SUDs. For example, NIDA has invested in research supporting the development of vaccines and antibodies for the treatment of SUDs. One example of NIDA’s efforts in this area is an ongoing collaboration with Selecta Biosciences to develop a novel nicotine vaccine. A lingering challenge that NIDA-supported research is working to address has been the development of vaccines that stimulate an immune response powerful enough to neutralize high concentrations of a drug before it enters the brain.
Budget Policy: The FY 2017 Budget estimate is $178.380 million, which is $0.458 million or 0.3 percent less than the FY 2016 Enacted level.
Program Portrait: Improving Adherence to Buprenorphine Treatment
FY 2016 Level: $9.0 million
FY 2017 Level: $9.0 million
Change: $0.0 million
Opioid abuse continues to be a major public health crisis in the United States; while some progress has been made in reducing the misuse of prescription opioid medications, use of heroin has rapidly increased in recent years. In 2014, an estimated 1.9 million Americans over the age of 12 had an opioid use disorder related to prescription opioids, and nearly 590,000 had an opioid use disorder related to heroin use – a nearly three-fold increase since 2007.3 NIDA is working closely with Federal partners through the HHS Opioid Initiative to combat opioid abuse and overdose through a multi-pronged approach that emphasizes improving prescribing practices, increasing access to the overdose reversal drug naloxone, and expanding access to medication-assisted treatment (MAT).
MAT – an approach that combines pharmacotherapy and psychosocial treatment – is the standard of care for the treatment of opioid use disorders. Buprenorphine is one of three FDA-approved medications that has been shown to be a safe and highly effective treatment for opioid addiction. NIDA has partnered with Titan Pharmaceuticals and Braeburn Pharmaceuticals to develop an implantable continuous delivery formulation of buprenorphine. This formulation, called Probuphine, would be implanted under the skin to deliver a steady dose of buprenorphine for six months, an innovation that improve the efficacy and acceptance of buprenorphine maintenance treatment by: 1) removing the need to take a daily pill, promoting continuous patient adherence; 2) preventing diversion of the drug; and 3) eliminating the risk of accidental ingestion by children. On September 28, 2015, FDA accepted a resubmission of the Probuphine New Drug Application (NDA) that includes results from a Phase III double-blind clinical study.
The development of Probuphine through this public-private partnership is illustrative of NIDA’s commitment to supporting innovations in SUD treatment that can effectively be brought to market. Widespread availability of safe, effective, and easily administered medications for addiction treatment is vital to reducing the serious harms, including overdose and death, that are associated with opioid use disorder.
Clinical Trials Network (CTN): CTN comprises 13 research nodes and more than 240 community treatment programs and/or medical settings in over 40 States plus the District of Columbia and Puerto Rico. Current initiatives are emphasizing research to develop and test strategies for the integration of SUD treatment into mainstream general medical settings, embedding research in clinical practice, and enhancing capacity to leverage electronic health record data in research studies. Through collaborations with clinical investigators, CTN generates research-based strategies needed for the integrated management of patients with substance misuse/SUD in general medical settings and linked specialty care treatment settings. CTN develops and tests the feasibility and effectiveness of interventions and health system approaches for SUDs and related disorders, such as co-occurring mental health disorders and HIV, in diverse patient populations. CTN is currently conducting studies evaluating: 1) a comparison of Vivitrol® (extended-release naltrexone) to Suboxone® (buprenorphine and naloxone) Sublingual Film for patients addicted to heroin or other opioids, including prescription pain relievers; 2) a combination therapy with Vivitrol® plus Wellbutrin XL® (bupropion hydrochloride, Extended-release Tablets) for treatment of methamphetamine addiction; 3) Vivitrol® for HIV-positive opioid users in HIV treatment settings; and 4) a brief screening and assessment instrument to identify patients with substance misuse and SUDs in general medical settings. Research under development includes several studies which will utilize electronic health record data from large healthcare systems to enable larger, more efficient research trials.
Budget Policy: The FY 2017 Budget estimate is $43.140 million, which is $0.111 million or 0.3 percent less than the FY 2016 Enacted level.
Intramural Research Program (IRP): IRP performs cutting-edge research within a coordinated multidisciplinary framework to: 1) elucidate the nature of the addictive process; 2) evaluate the potential of emerging new therapies for SUDs, both pharmacological and psychosocial; and 3) describe the long-term consequences of drugs of abuse on systems and organs, with particular emphasis on the brain and its development, maturation, function, and structure. For example, IRP is furthering SUD research by collaborating with pharmaceutical industry partners to study a potential medication that can decrease methamphetamine craving. In addition, IRP is working to understand the impact of long lasting deficits in the prefrontal cortex – an area of the brain that mediates decision making – caused by cocaine and heroin use. In an animal model, scientists can reverse this deficit by hyper-stimulating the prefrontal cortex for brief periods. This intervention is being developed as a possible therapy for addiction. IRP is also working to develop clinically useful indicators (biomarkers) of nicotine addiction severity or treatment efficacy. Scientists are using brain imaging along with genetic and epigenetic data to develop quantitative addiction markers that will support the development of more efficacious treatments and discovery of novel treatment targets. IRP scientists are also working to better understand factors that contribute to cravings and relapse. Memories of items, people, or environments that are present when addicted individuals take drugs become powerful cues that trigger them to relapse again and again. Scientists have shown that these memories are stored in specific patterns of neurons called neuronal ensembles in the brain. Researchers have been successful in inactivating these drug-related ensembles and memories in animal models, and are developing similar procedures that might be used in humans to selectively impair harmful addiction memories. In addition, IRP scientists are developing a mobile health toolbox to collect data on the daily-life reality of addiction. These tools can support intensive assessments to help identify individual and environmental influences on drug craving and use to understand when people are most vulnerable to relapse. One of the goals of this research is to deploy a mobile intervention that will automatically predict imminent drug use and deliver help just when a person needs it.
Budget Policy: The FY 2017 Budget estimate is $91.657 million, which is $1.354 million or 1.5 percent more than the FY 2016 Enacted level.
Research Management and Support (RMS): RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. Additionally, the functions of RMS encompass strategic planning, coordination, and evaluation of NIDA’s programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public. NIDA currently oversees more than 1,600 research grants and more than 100 research and development contracts. In addition to the infrastructure required to support research and training, NIDA also strives to provide evidence-based resources and educational materials about SUDs and to raise awareness of the science relating to cutting-edge issues such as opioid overdose prevention, marijuana research, synthetic drug trends, and Medication Assisted Treatments.
Budget Policy: The FY 2017 Budget estimate is $63.862 million, which is $0.944 million or 1.5 percent more than the FY 2016 Enacted level.
Education and Outreach: NIDA is committed to working with broad range of stakeholders including scientific, professional, patient, and family organizations that comprise the substance abuse field. We partner on a variety of efforts to widely disseminate scientific information to support the successful implementation of evidence-based practices.
Healthcare providers are a key target audience for NIDA’s outreach efforts. NIDA leads the NIH Pain Consortium Centers of Excellence in Pain Education (CoEPEs); these 12 centers work to enhance patient outcomes by improving the education of healthcare professionals about pain and its treatment. The CoEPEs act as hubs for the development, evaluation, and distribution of pain management curriculum resources for medical, dental, nursing, and pharmacy schools to improve how health care professionals are taught about pain and its treatment. In addition, NIDA supports an initiative known as NIDAMED to develop resources to educate healthcare providers on issues related to substance use and addiction and to increase awareness of the impact of substance use on patient health. Through this project, NIDA is currently creating multiple continuing medical education (CME) online modules that focus on: 1) prescription opioids; 2) marijuana; 3) screening for substance use; 4) key messaging to communicate to adolescents and their caregivers about drugs; 5) successful ways for clinicians to engage in conversations with adolescents (ages 13-18) and their parents; and 6) how best to address issues such as privacy and confidentiality. CME will also create clinician/patient communication tools, which may include brochures/handouts or an in-office, mobile ready game or app that clinicians can use with adolescents to help initiate a conversation about substance use and provide information about the consequences of use. This initiative aims to train 28,000 clinicians in prevention of SUDs in adolescents by October 2019.
NIDA also is committed to being at the forefront of training the next generation of innovative researchers by supporting both pre-doctoral and postdoctoral-level scientists interested in drug abuse research. These efforts also focus on increasing the number of underrepresented scholars and researchers, including women and minorities, actively participating in drug abuse research through our outreach, and sponsored career development and research training opportunities.
References
- U.S. Department of Health and Human Services. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, CDC; 2014. Available at http://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf (PDF, 36MB)
- Results from the 2014 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2015.
- http://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2014/NSDUH-DetTabs2014.pdf (PDF, 10MB)