Organizational Chart
Appropriations Language
For carrying out section 301 and title IV of the Public Health Services Act with respect to drug abuse [$1,059,848,000] $1,094,078,000 (Public Law 111-117, Consolidated Appropriations Act, 2010)
Tables
- Amounts Available for Obligation Table
- Appropriations History Table
- Authorizing Legislation Table
- Budget Authority by Object Table
- Budget Authority by Program Table
- Budget Mechanism Table
- Detail of Full-Time Equivalent Employment (FTE) Table
- Detail of Positions Table
- New Positions Requested Table
- Salaries and Expenses Table
- Summary of Changes Table
Major Changes in the Fiscal Year 2011 Budget Request
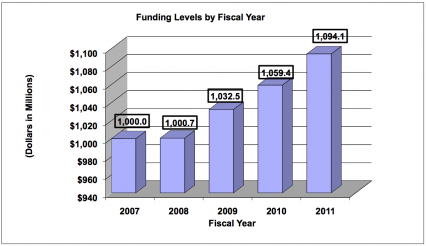
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2011 budget request for NIDA, which is $34.6 million greater than the FY 2010 Estimate, for a total of $1.094 billion.
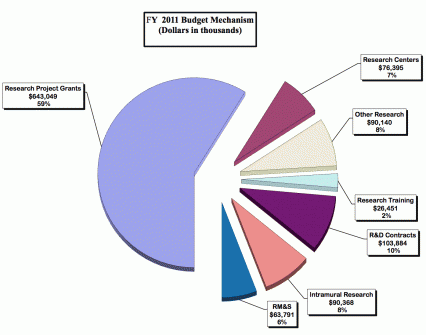
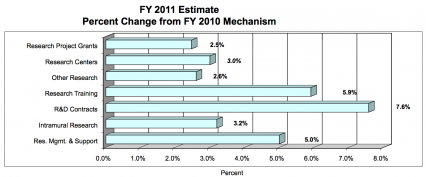
Research Project Grants (+$15.489 million; total $643.049 million): NIDA will support a total of 1,443 Research Project Grant (RPG) awards in FY 2011. Non-competing RPGs will increase by 28 awards and increase by $17.4 million. Competing RPGs will decrease by 15 awards and decrease by $2.8 million. Within the total, NIDA will provide increases for the Director's scientific priorities, which include genomics and other high-throughput technologies, translational medicine, and initiatives that benefit health care reform and reinvigorate biomedical research.
Research Training (+$1.476 million; total $26.451 million): NIDA will continue its support for the research training program by providing an increase of $1.274 million to fund a six percent increase in average stipend levels.
Intramural Research (+$2.802 million; total $90.368 million): The request will help offset the cost of pay and other increases. NIDA will work to identify areas of potential savings within the Intramural Research Program that will allow the institute to continue to achieve its program goals and accomplishments.
Research Management and Support (+$3.038 million; total $63.791 million): NIDA oversees almost 1,800 research grants, more than 500 full-time training positions, and over 200 research and development contracts. The increase will be used to partially offset the expenses associated with pay raises and other cost increases necessary to provide for the effective administrative, planning and evaluation, public information and communications, and scientific leadership of the institute.
Budget Graphs
History of Budget Authority and FTE's
Distribution by Mechanism
Change in Selected Mechanisms
Justification of Budget Request
Authorizing Legislation: Section 301 and title IV of the Public Health Service Act, as amended.
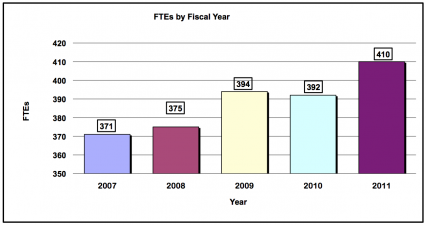
| FY 2010 Omnibus | FY 2010 Appropriation | FY 2011 President's Budget | FY 2011 +/- 2010 Omnibus | |
|---|---|---|---|---|
| BA | $1,032,457,000 | $1,059,446,000 | $1,094,078,000 | +$34,632,000 |
| FTE | 394 | 392 | 410 | +18 |
This document provides justification for the Fiscal Year (FY) 2011 activities of the National Institute on Drug Abuse (NIDA), including HIV/AIDS activities. Details of the FY 2011 HIV/AIDS activities are in the "Office of AIDS Research (OAR)" Section of the Overview. Details on the Common Fund are located in the Overview, Volume One. Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural; and Other.
Director's Overview
Drug abuse and addiction continue to drive high rates of morbidity and mortality as well as exact an exorbitant social and economic cost. However, the landscape is rapidly changing: paradigm shifts in our scientific understanding of how drugs affect the brain, and their myriad health consequences, including addiction, are allowing us to translate a dramatically improved knowledge into the next generation of effective prevention and treatment interventions.
Gains in translation
The success of these efforts hinges on our ability to expand the two-way streets connecting bench with bedside. This is why NIDA is committed, for example, to link recent and stunning advances in genomics (e.g., the ability to analyze entire individual genomes) with emerging technologies for characterizing, with unprecedented detail, other critical domains, including environmental factors, brain structure, pre/perinatal clinical histories, and epigenetic marks (chemical alterations to the DNA, triggered by a host of environmental factors, that can affect gene expression and function). The resulting linkage maps are much more than academic exercises: the unprecedented level of resolution and multilevel integration that can be achieved by these maturing datasets will help us uncover important risk and resiliency mechanisms as well as new molecules and circuits that could be targeted by novel pharmaco/behavioral therapeutics.
Still, we face lingering challenges, such as the obstacles to pharmaceutical industry engagement in developing medications for addiction. In spite of this problem, NIDA continues its efforts to bring new medications forward by identifying promising compounds and supporting their development. Towards that end NIDA is planning to issue a funding announcement for a translational Avant-garde award to further research that helps the transition from the drug discovery stage to pre-clinical and clinical phases. Another important initiative in this context is the development of immunotherapies (e.g., vaccines) for the treatment of nicotine, cocaine, and methamphetamine addictions. The vaccines stimulate the body to produce antibodies that bind a drug while it is still in the bloodstream, thereby reducing its entry into the brain and blocking its pharmacological/behavioral effects. This strategy taps into a well-established model of harnessing the healing powers of our own bodies, a first, for addiction medicine.
Reform with Access in mind: a top priority
The development of new and better interventions will not result in significant progress unless we also leverage the best science in the pursuit of a meaningful health reform that sets available and affordable access to those treatments as top priorities. From our Institute's point of view, a critical element to improving care for substance abuse is to gain health care providers' embrace of the notion that addiction is a disease that plays a role in many other ailments. Getting physicians invested in detecting substance use early, preventing escalation to abuse and addiction, and referring patients in need of treatment can greatly improve our chances of closing the treatment gap. Towards that end, NIDA has recently deployed NIDAMED, a physician outreach program that includes user friendly online tools designed to help primary-care physicians screen their patients for alcohol, tobacco and other drug use.
A second requirement for this transformation will be to achieve a much better understanding of how to deliver the most effective interventions to those who need them, and reduce the health disparities that affect Americans. In this context, we pay particularly close attention to the special circumstances and pressing needs of the most vulnerable and underserved among us-including children and adolescents, minorities, prisoners or others involved in the criminal justice system, and people with HIV-testing targeted interventions in real-world settings through NIDA's Drug Abuse Treatment Clinical Trials Network and Criminal Justice-Drug Abuse Treatment Studies.
NIDA's approach to reduce the burden of drug addiction involves an unrelenting focus on the potential to translate research into transformative clinical outcomes through initiatives like "seek and treat," which will evaluate and garner acceptance of screening and treating both drug abuse and HIV within the criminal justice system. Also relevant in this context are NIDA's international HIV prevention and treatment efforts, which will continue to build on past successes, like the development and increasing rates of adoption of buprenorphine for sustaining heroin abstinence, and preventing the spread of infectious diseases.
Reinvigorating and empowering the biomedical research community
One of the consequences of rapid scientific advances is a palpable excitement, within lay and research circles alike, about discovery possibilities that lie in future research. Thus, this point in time presents an excellent opportunity to reinvigorate and empower not only the biomedical research community, but also the general population, from which will emerge the future cadre of science leaders. NIDA has partnered with other NIH Institutes, under the umbrella initiative known as NIH Blueprint, to offer multidisciplinary training opportunities that present avenues for motivating interest in innovative brain research that could impact a variety of neurological/psychiatric disorders. Additionally, NIDA participates in the Intel International Science and Engineering Fair (Intel ISEF), selecting three projects each year to receive awards for exemplary work in Addiction Science.
Training is of course a critical component of a proactive and modern research community. NIDA has partnered with other NIH Institutes, under the umbrella initiative known as NIH Blueprint, to offer multidisciplinary training opportunities that present additional avenues for motivating interest in innovative brain research that could impact a variety of neurological/psychiatric disorders.
Overall Budget Policy
The FY 2011 request for NIDA is $1.094 billion, an increase of $34.6 million or +3.3 percent over the FY 2010 enacted level. NIDA will continue to support new investigators and to maintain nearly the same number of competing RPGs as in FY 2010. In FY 2011, NIDA will support new investigators on R01 equivalent awards at success rates equivalent to those of established investigators submitting new R01 equivalent grants. Research priorities include those that position the Institute to optimally benefit from scientific advances able to transform our progress in preventing and treating drug abuse and its consequences and thereby further NIDA's public health mission. NIDA will support research that uses innovative genetics tools and technologies, furthers development of promising medications, and that translates the results of evidence-based findings to improve drug abuse interventions and promote greater access to them worldwide. The Institute also seeks to maintain a balance between solicitations issued to the extramural community and funding made available to support investigator-initiated projects. Intramural research and research management and support receive increases to help offset the cost of pay and other costs. In addition, funds are included in R&D contracts to support several trans-NIH initiatives, such as the Therapies for Rare and Neglected Diseases program (TRND), the Basic Behavioral and Social Sciences Opportunity Network (OppNet), and support for a new synchrotron at the Brookhaven National Laboratory, as well as increased support for other HHS agencies through the program evaluation set-aside.
FY 2011 Justification by Program
Program Descriptions and Accomplishments
Basic and Clinical Neuroscience: Basic and Clinical neuroscience represent two programs in NIDA that work together to expand understanding of the neurobiological, genetic/epigenetic, and behavioral factors underlying drug abuse and addiction. Specifically, they examine the factors affecting increased risk and/or resilience to drug abuse, addiction, and drug-related disorders; the mechanisms of addiction; and the effects of drugs on the brain and behavior. Collectively, this research provides the fundamental information to develop and communicate prevention and treatment interventions for drug abuse and addiction.
Budget Policy: The 2011 estimate for this program area is $519.497 million. By applying funds from grants that are ending in FY 10, we will pursue opportunities in line with our mission to study drug abuse and its consequences across the lifespan, from birth to youth to old age. Because adolescents are a key population of interest, a FY 10 RFA will award grants that integrate findings from research on brain development, cognition, and neuroscience into the creation of innovative and effective, drug abuse treatments targeted for youth. Results should enhance our understanding of adolescent neurodevelopment, including normal cognitive growth, executive function impairments stemming from drug abuse, and the specific thinking capacities that are necessary for treatments to be efficacious, thereby improving our ability to tailor treatments to individual needs and capabilities. Another FY 10 RFA will support studies proposing to use deep sequencing technologies to identify the specific genetic variants that affect addiction risk in well-characterized drug abusing population samples. Genome-wide association studies (GWAS) have identified genomic regions associated with addiction phenotypes, providing opportunities for further refinement using this deep sequencing approach.
Epidemiology, Services and Prevention Research: This program area seeks to promote integrated approaches to understand and address the interactions between individuals and environments that contribute to the continuum of drug abuse-related problems. This NIDA division supports research and major data collection systems, as well as surveillance networks. Program efforts help identify substance abuse trends locally, nationally, and internationally; guide development of responsive interventions for a variety of populations; and encourage optimal service delivery in real-world settings.
Reducing HIV Transmission and Improving Drug Treatment in the Criminal Justice System
More than 7 million people in the U.S. are involved with some aspect of the criminal justice system—2.3 million in prisons or jails, and about 5 million under community-based supervision. The drug abuse, mental health, and HIV treatment needs of this population are tremendous: approximately half of State and Federal prisoners meet criteria for alcohol or drug addiction, with about 22,000 State and Federal inmates known to be infected with HIV or to have confirmed AIDS at the end of 2006—a prevalence rate roughly 3 times that of the general U.S. population. This also makes criminal justice settings opportune venues for identifying and treating HIV and drug use disorders among high-risk populations and for intervening to counter the relapse-recidivism cycle.
As part of our approach to address this cluster of problems, which present both public health and safety issues, NIDA supports the Criminal Justice-Drug Abuse Treatment Studies (CJ-DATS) Initiative- multisite research collaborative, launched in 2002 with several partners to develop and test evidence-based approaches for treating drug abuse and related conditions in the criminal offender population. Now in its second phase, this expanded initiative will test implementation strategies to foster treatment adoption and promote continuing care. For it is into the community where the vast majority of all inmates are eventually released and where, unfortunately, even those who received adequate drug abuse and/or HIV treatment while imprisoned, fail to maintain it.
NIDA plans to test a new strategy in this regard, aimed particularly at improving HIV outcomes for criminal justice populations before and after release. This approach, termed "Seek, Test, and Treat," involves reaching out to high-risk, hard-to-reach groups who have not recently been tested (seek), providing HIV testing (test), and initiating, monitoring, and maintaining HAART therapy for those who test positive (treat). It also involves providing drug abuse treatment, without which individuals are unlikely to comply with their antiviral medications. NIDA hopes this initiative will not only expand access to HIV testing for those in the criminal justice system, but will improve the provision and maintenance of HAART following community reentry, when treatment lapse and viral load rebound can heighten risk of HIV transmission. Hoped-for studies will identify and address ways that criminal justice and public health entities can better coordinate their efforts to successfully maintain treatment for HIV-positive offenders during and after community reentry.
Budget Policy: The 2011 estimate for this program area is $260.465 million. A major focus for this NIDA program area is to improve drug abuse prevention and treatment services among populations in need. For example, through a FY 10 RFA, NIDA will support research aimed at improving HIV outcomes for criminal justice populations pre- and post-release. Another FY 10 RFA is encouraging studies on the associations between drug abuse, deployment stress, and combat trauma among U.S. military personnel and their families. Exposure to combat has been linked with increased substance abuse risk, as well as post traumatic stress and depressive disorders and disrupted social relationships. Resulting research should help identify risk and protective factors, develop and test substance abuse prevention and treatment interventions, and explore the utility of existing evidence-based prevention interventions and services for substance abuse alone or with comorbid conditions across the deployment cycle for military personnel, veterans and their families.
Pharmacotherapies and Medical Consequences: This program area is responsible for medications development aimed at helping people recover from drug abuse and addiction and sustain abstinence. It capitalizes on research showing the involvement of different brain systems in drug abuse and addiction, beyond the dopamine system, to develop medications in response to a variety of newly defined targets. This program area also seeks solutions addressing the medical consequences of drug abuse and addiction, including infectious diseases such as HIV.
Medication innovations for treating drug abuse and addiction
NIDA responded to Congress' call to "treat the symptoms and disease of drug abuse" by establishing in 1994 a program to support the discovery and testing of new addiction medications. Since that time, breakthrough discoveries have engendered a profound transformation in our understanding of the mechanisms and consequences of drug abuse and addiction, offering a unique opportunity for development of new therapies to help alleviate the devastating personal and social impacts of addiction.
One exciting new approach draws from a centuries-old practice to combat disease. Vaccination, which harnesses the body's own immune system to counter a broad range of disease agents, is the being explored to improve the effectiveness of addiction treatments. Briefly, an anti-drug vaccine triggers the production of antibodies that then seek and bind the specific drug in the bloodstream, preventing its entrance to the brain, thus blocking its pharmacological and behavioral effects. This immune-based strategy has been linked with decreased drug use in patients who produced high levels of antibodies against cocaine or nicotine. Should the vaccine approach prove successful, it would represent a stunning breakthrough that could enhance the impact of existing therapies, particularly in the case of cocaine addiction, for which no medications are currently available. Cessation programs for nicotine addiction would also benefit, since vaccines could assist in curbing the exceedingly high relapse rates among quit attempters.
NIDA also continues to capitalize on our greater understanding of the neurobiology underlying addiction and of newly identified candidate systems and molecules, applying advances in genetics, brain imaging, neurochemistry, and molecular biology to hone research on medications development. Because no medications exist for stimulant, cannabis, or polydrug addiction, these areas remain priorities, along with finding new ways of reducing addiction liability and diversion and improving treatment adherence through alternative formulations and delivery methods. Pharmaceutical companies have been reluctant to invest in addiction medications, largely because of perceived financial disincentives and stigma; thus NIDA remains one of the only entities addressing this critical gap and major public health need.
Budget Policy: The 2011 estimate for this program area is $116.017 million Program plans for 2011 give priority to a collaborative product development partnership (PDP) to develop, test, and facilitate the distribution of safe and effective medications for the treatment of tobacco dependence. The goal is to involve public (government agencies and institutes), non-profit (academia, NGOs, philanthropic institutions), and private sector entities to accelerate the development and production of smoking cessation drugs at a reasonable cost. NIDA will also continue to stimulate research to develop medications for the treatment of cocaine, methamphetamine, and/or cannabis addictions, for which there are no FDA-approved medications currently available. A FY 10 RFA will encourage studies to identify promising medications for cannabis-use disorders, as well as their medical and psychiatric consequences. Basic and clinical research will be supported to assess the safety and efficacy of candidate treatments. Another FY 10 RFA will support research on the design, synthesis and screening of novel compounds that affect high priority targets for addiction medications, for which no selective or developable molecule currently exists.
Clinical Trials Network: NIDA's National Drug Abuse Treatment Clinical Trials Network (CTN), which now comprises 16 research nodes and more than 240 individual community treatment programs, serves 34 States, plus the District of Columbia and Puerto Rico. The CTN works to develop treatment protocols for drug abuse and addiction and related conditions, such as comorbid mental health disorders and HIV, testing the real-world effectiveness of promising medication and behavioral treatment approaches with diverse patient populations and community treatment providers. It also serves as a research and training platform to help NIDA respond to emerging public health areas. Currently, the CTN provides a research platform for more than 30 research grants and a training platform for 60+ research fellows and junior faculty.
Budget Policy: The 2011 estimate for this program area is $43.940 million. Program plans, along with expected accomplishments, are a culling and analysis of data for initiatives begun in FY 09 to (1) assess the effectiveness of a 12-step facilitation intervention for stimulant abusing patients in initiating and sustaining their involvement with support groups like Cocaine or Alcoholics Anonymous, (2) determine whether adding individual drug counseling to buprenorphine/naloxone (BUP/NX) treatment, along with Standard Medical Management (SMM), improves outcomes for patients addicted to pain medications, and (3) compare the effect of BUP/NX versus methadone on liver enzymes in patients entering opioid treatment programs, a phase 4 study requested by the FDA to provide additional information on risks, benefits, and optimal use of these medications. To test and validate more such effective and efficient treatments and facilitate their adoption by treatment providers nationwide, the CTN is seeking in FY 10 new cooperative agreement applications and renewal applications from established clinical investigators to participate in the CTN for the next 5 years.
Intramural Research Program (IRP): This Intramural program performs cutting edge research within a coordinated multidisciplinary framework. The IRP attempts to elucidate the nature of the addictive process; to determine the potential use of new therapies for substance abuse, both pharmacological and psychosocial; and to decipher the long-term consequences of drugs of abuse on brain development, maturation, function, and structure, and on other organ systems. In addition, the IRP supports an HIV/AIDS Pathophysiology and Medications Discovery Program, which focuses on (1) how HIV or its products cross the blood-brain barrier, (2) how toxic compounds generated by HIV invade brain cells, and (3) the development of compounds to block the toxic effects of HIV on immune system cells.
Budget Policy: The 2011 estimate for this program area is $90.368 million. NIDA has made major strides by embracing new techniques, including new tools to measure neighborhood-level environmental risk factors and the effect of psychosocial stress on individuals with substance-use disorders by collecting behavioral and physiological data in participants' real-time environments. This activity, now well underway and attracting considerable interest from addiction researchers worldwide, represents the first systematic, prospective effort to link indices of community-level risk to intensive field measurements of individual attempts at behavior change.
Research Management and Support (RMS): RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. Additionally, the functions of RMS encompass strategic planning, coordination, and evaluation of NIDA's programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public. NIDA currently oversees more than 1,800 research grants and more than 190 research and development contracts. In addition to the infrastructure required to support research and training, NIDA also strives to educate the public about drug abuse and addiction and to raise awareness of the science behind it.
Budget Policy: The 2011 estimate for this program area is $63.791 million. NIDA will continue to support scientific meetings to stimulate interest and develop research agendas in areas significant to drug abuse and addiction. NIDA will also continue to support educational outreach aimed at various audiences, including HIV high-risk populations, physicians, courtroom judges, and educators to promote awareness of substance abuse issues and disseminate promising prevention and treatment strategies. Adolescents represent a key target audience for our education efforts. Building upon NIDA's popular Drug Facts Chat Day, in which students from across the country are able to learn the facts about drug abuse from NIDA scientists, in 2010 NIDA plans to hold a "teen awareness week" with Chat Day at its center, and other events scheduled nationwide to arm teens with information that can help them make healthy decisions.
NIH Common Fund: NIDA is the co-lead, with NIEHS and NIDCD on an RFA on Technology Development in Epigenetics. Its goal is to foster the development of revolutionary technologies with the potential to significantly change how epigenomics research is performed in the future. This could enable the use of epigenomic changes to diagnose and investigate the effects of environmental exposures (e.g., drugs of abuse, toxins, infection) on disease (e.g., cancer, neuropsychiatric disorders, aging). NIDA also participates in the support of Institutional Training Grants focused on Interdisciplinary training through the NIH Blueprint and the NIH Common Fund. The purpose of these initiatives is to foster changes in academic culture as well as interdisciplinary team approaches to research, with training provided to investigators at differing career stages.
Recovery Act Implementation
Recovery Act Funding: $261.156 million
In FY 2009, NIDA received about $261.156 million under the Recovery Act. Of this amount, $136 million was obligated in FY 2009 and $125 million will be obligated in FY 2010. This boost in funding will speed the pace of research, provide jobs, and advance the science needed to address addiction and its related health consequences. Studies will encompass genetic and other risk factors, neighborhood-specific prevention approaches, novel medications to treat addiction, and comparisons and costs of translating effective strategies into community and criminal justice settings. Findings will help our Nation counter one of its costliest, preventable, and treatable health problems. NIDA has designated three Signature Areas as key subsets of this research:
- Eradicate tobacco addiction: Nicotine addiction remains at unacceptably high levels and is exorbitantly expensive. Recent scientific advances position us to achieve this important goal through support of research to develop effective therapeutic and prevention interventions.
- Genetic influence on the development and structure of the human brain: Research will advance our understanding of the interplay between genes and environment in shaping brain development, elucidating the contribution of specific genes to neuropsychiatric disorders and how environmental factors can trigger disease in those genetically vulnerable.
- Research and development of anti-drug vaccines: Antibodies can be generated against specific drugs of abuse in order to reduce their entry into the brain and block their behavioral effects. If successful, this novel approach would represent a major breakthrough that could greatly enhance the impact of existing addiction therapies.
To address two of our signature areas, NIDA used ARRA funds to award $10 million grant to Nabi Biopharmaceuticals (Nabi) to advance the development of a nicotine vaccine and move it closer to final FDA approval. As a result of ARRA funding, Nabi has entered an agreement with GlaxoSmithKline to provide an additional $40 million to exclusively in-license NicVAX on a worldwide basis and develop follow-on, next-generation nicotine vaccines. This work is an excellent example of leveraging government resources to further develop and market a medication for tobacco addiction.