Congressional Justification
| Authorizing Legislation: | Section 301 of the Public Health Service Act, as amended. Reauthorizing legislation will be submitted. | ||||||
| Budget Authority: | |||||||
| FY 2000 Actual | FY 2001 Estimate | FY 2002 Estimate | Increase or Decrease | ||||
| FTEs | BA | FTEs | BA | FTEs | BA | FTEs | BA |
| 368 | $696,292,000 | 378 | $780,975,000 | 387 | $907,369,000 | 9 | $126,394,000 |
This document provides justification for the Fiscal Year 2002 activities of the National Institute on Drug Abuse (NIDA), including HIV/AIDS activities. A more detailed description of NIH-wide Fiscal Year 2002 HIV/AIDS activities can be found in the NIH section entitled "Office of AIDS Research (OAR)."
Introduction
A virtual explosion of research advances over the past decade is significantly changing how we as a Nation prevent, treat, approach, and even discuss drug abuse and addiction. NIDA supports over 85 percent of the world's research on all drugs of abuse, both legal and illegal, with the exception of a primary focus on alcohol. NIDA also has a comprehensive program on the medical health and developmental consequences of drug abuse, the effects of drug use on other diseases, and the multidisciplinary aspects of HIV/AIDS.
Recognizing that injection drug users and their sex partners now account for more than half of all new HIV infections annually, NIDA supported researchers are working to better understand the dynamic patterns in the transmission of HIV and other infectious diseases in drug using populations, to develop effective behavioral risk reduction interventions, and to reduce the morbidity and mortality associated with HIV/AIDS. NIDA has made the dissemination of these findings a priority, particularly to international communities that are being devastated by this disease. Because HIV infection rates are increasing in the adolescent population, particularly among minority females, NIDA is expanding its portfolio to address the particular challenges and needs of young people. In short, NIDA researchers are making great progress in shedding new light on how best to prevent and treat the spread of intertwined infectious diseases and drug abuse epidemics. Advances continue to come at an extraordinary rate from throughout NIDA's entire portfolio.
One area of major focus and advance has been drug abuse treatment. A little over a year ago, NIDA established the National Drug Abuse Treatment Clinical Trials Network (CTN) which now includes 14 university-based research centers and over 80 community-based drug treatment programs working in partnership to move science-based treatments into real life settings. A unique feature of the CTN is the involvement of the community treatment programs (CTPs). These CTPs represent a broad array of patient populations and treatment approaches. They are integrally involved in all aspects of the CTN, from the design of the protocols to the implementation of the treatment. Their successes are truly noteworthy. In its first year, the CTN developed and trained participants to implement the first seven protocols, patients are already being recruited. Five additional protocols will likely begin enrolling patients in the Fall of 2001.
By forging partnerships between researchers and CTPs who are serving on the frontlines of the battle against drug abuse and addiction, the CTN will engage the entire drug abuse community in a national effort against addiction and its consequences. Additionally, the CTN provides a much needed infrastructure to more efficiently and rapidly disseminate new research findings to practitioners and patients across the country.
Another example of how NIDA supported research is benefitting the citizens of this Nation can be seen by peering into the clinician's toolbox. NIDA-supported science has brought to fruition the first new anti-addiction medications for treating heroin addiction in nearly a decade. Not since LAAM (levo-alpha-acetyl-methadol) was approved in 1993 have new medications been developed for treatment of opiate dependence. Building on the substantial body of research on the neurochemical details of how opiates produce their analgesic and behavioral effects, NIDA has been able to develop, in collaboration with a pharmaceutical company, the partial agonist buprenorphine. Buprenorphine has been shown to be a safe and effective medication for treating opiate addiction that is long-lasting, well tolerated by addicts and when combined with naloxone has very limited diversion potential. In fact, it is thought to be so safe and effective that Congress and the President took the historic step of passing legislation that allows qualified physician's to prescribe certain anti-addiction medications in an office setting, including buprenorphine and buprenorphine/naloxone, once approved by FDA. This step not only advances drug addiction treatment practice, but also research. This will allow addiction treatments to be put on an equal footing with treatments for other diseases, moving us toward a less restrictive environment for dispensing narcotic addiction treatments. Buprenorphine products are truly unique medications that will expand treatment access to previously inaccessible populations. (See "Story of Discovery" section)
Treatment is not the only area where NIDA success stories abound. Powerful new research tools continue to be developed that are proving invaluable to scientists unraveling the neurobiological complexities that underlie drug abuse and addiction. Functional neuroimaging techniques, such as positron emission tomography (PET), single photon emission computed tomography (SPECT), and functional magnetic resonance imaging (fMRI), along with electroencephalography (EEG), provide windows through which we can observe the working brain as patients experience a variety of drug abuse-related phenomena. With these imaging techniques, we have the ability to view the changes that occur in the brain as drugs exert their psychoactive effects. These new tools are also teaching us about long-lasting changes in brain structure and function that drugs such as methamphetamine and MDMA (3,4-methylenedioxy-methamphetamine), or ecstasy, can have on the brain. These changes may persist years after the individuals have stopped using the drugs. In a recent study neuroimaging was performed in over two dozen methamphetamine abusers who were drug free for up to 21 months. There was a significant reduction of a neurochemical marker called N-acetylaspartate (NA) that determines a cell's viability in several brain areas. This reduction of NA, in addition to other brain abnormalities found, indicates that neurons in these regions have been damaged.
Through the use of cutting-edge molecular techniques researchers are also learning more about what causes individual differences in vulnerability to addiction. It appears to be a combination of genetic and environmental input, with the genetic portion accounting for anywhere from 30 to 80 percent depending on the drug. Given the completion of the human genome project and the advances in technology that have led to the identification of genes contributing to many complex diseases, including diabetes, asthma, and cardiovascular diseases, there is now reasonable optimism that researchers will be able to find genes that influence complex bio-behavioral diseases such as addiction. In fact, recent findings are enabling us to pinpoint specific genes that may be involved in addiction to certain drugs. For example, researchers found that individuals with a genetic deficiency in an enzyme that metabolizes nicotine (CYP2A6) are less likely to start smoking, and smoke less if they do start, than individuals with normal CYP2A6 activity. Building on this knowledge, researchers tested more than 200 compounds to decrease CYP2A6 activity and found that one compound (methoxsalen) commonly used to treat skin disorders may be helpful to people who want to quit smoking.
With all of these advances comes increasing responsibility to translate these findings in ways that can be used by prevention and treatment practitioners, patients, and their families. To meet this challenge, NIDA and its stakeholders underwent two major strategic planning exercises in the past year to decide how best to accomplish this. Through efforts outlined in NIDA's "Five Year Strategic Plan: Bringing the Power of Science to Bear on Drug Abuse and Addiction" and "NIDA's Strategic Plan on Reducing Health Disparities," NIDA is intensifying efforts to significantly reduce drug abuse and addiction and their behavioral, health, and social consequences. Much of this will be accomplished by the continuous support and development of NIDA's diverse research portfolio and a commitment to share these research findings with the broadest community possible. NIDA has also initiated an intensive Blending of Clinical Research and Practice Research Initiative to ensure the translation of findings. NIDA recognizes that the scientific knowledge generated through NIDA research is a critical element to improving the overall health of the Nation. Our goal is to ensure that science, not ideology or anecdote, forms the foundation for all of our Nation's drug abuse reduction efforts. Some areas of focus identified through our strategic planning processes follow in the new activities section of this document.
New Initiatives and Priority Areas for Drug Abuse Research
Improving the Quality of Drug Abuse Treatment Nationwide. NIDA has declared as its foremost goal for the new millennium the improvement of drug abuse treatment nationwide using science as the vehicle. A number of activities are involved in this endeavor, including the development of effective medications and behavioral therapies, expansion of the national infrastructure that NIDA has put in place to test these new treatments in real life settings, an increased understanding of the organizational and financing impediments to drug treatment, and supporting research that best determines how to treat addicted criminal offenders.
The development of new pharmacological and behavioral treatments will remain a high priority for the Institute, especially for stimulants such as cocaine and methamphetamine, emerging club drugs, and drugs such as heroin and nicotine that continue to be used at unacceptable rates. Through NIDA's Medications Development Program, more than 60 compounds are being tested as potential treatments for addiction. A wide variety of behavioral treatments are also being tested in a variety of settings as stand-alone treatment therapies and as adjuncts to medications. NIDA has several promising compounds such as disulfiram, selegeline, and amantadine for treating cocaine addiction in various stages of multisite clinical trials. NIDA also has several promising methamphetamine treatments being tested in sites across the country, as well as a number of activities to develop immunotherapies or vaccines for addictions such as nicotine. NIDA will continue to apply powerful new computerized drug development techniques to all of these important undertakings. Several behavioral treatments, including ones that focus on motivational incentives and aftercare, are about to begin accruing patients for clinical trial protocols.
NIDA will continue to expand the national research infrastructure that it has established for moving science-based drug addiction treatments into real-life practice settings. The National Drug Abuse Treatment Clinical Trials Network (CTN) is based on a model used successfully by other NIH institutes. The Network's prime mode of operation is to deliver and test an array of behavioral and pharmacological treatments and to determine the conditions under which novel treatments are successfully adopted. In FY 2002 NIDA hopes to increase the size of the Network to include approximately 160 treatment programs in this consolidated research process. NIDA's plan is to have 10 to 20 research protocols, shown to be effective in laboratory settings or suggested by practitioners in the field, being tested in a wide variety of treatment settings across the country. Those programs, in turn, will be providing treatment to diverse patient populations including those of different ethnic and racial origins and gender preferences, adolescents, pregnant women, and poly-drug using individuals.
Combating Intertwined Epidemics and Increasing our Knowledge About the Medical Consequences of Drug Abuse. NIDA will continue to expand research on the full spectrum of health issues associated with drug abuse including the strong connection between drug use and other diseases, including HIV/AIDS, hepatitis B and C, tuberculosis, sexually transmitted diseases, and co-occurring illnesses such as depression, schizophrenia, and other disease manifestations. Research has shown that the diseases of addiction and HIV/AIDS, for example, are intimately intertwined epidemics that need to be addressed simultaneously with a wide variety of approaches. Hepatitis C is fast becoming another disease that needs to be addressed in an integrated public health strategy. Additionally, NIDA will focus on studying the natural history of a lifetime of drug abuse. Like other chronic diseases, it is important that we know far better than we do now the long-term medical and health consequences for individuals who are long-term drug abusers. NIDA will significantly expand studies of the effects of chronic drug abuse on general morbidity, neuropsychosocial, neurological, cardio-renal, immune, and other physiological systems.
Eliminating Health Disparities. Minority populations are disproportionately affected by the consequences of drug abuse. NIDA has developed a strategic plan to use science to significantly reduce these disparities. In implementing this plan, NIDA will expand efforts to understand the causes of and contributing factors to these inequalities and to ensure that minority issues and populations are adequately represented both in NIDA's comprehensive research portfolio and in the research communities. The strategic plan is part of a broader NIH-wide effort to eliminate health disparities in racial and ethnic groups. Some of the specific areas NIDA will address in the upcoming year include developing improved measures and designs to more appropriately assess drug abuse and addiction and related behaviors in racial/ethnic populations. NIDA will also focus more intensively on building the minority research infrastructure by increasing the clinical training experiences of minority students.
Responding to Emerging and Changing Drug Use Trends. All drug problems are fundamentally local in character. Thus there is a great need to understand the exact nature of drug trends within local communities and respond to them with locally appropriate strategies. Like a virus that mutates, both regional and national drug abuse patterns rarely remain static. Tried and true approaches may not work with many of the new drugs that are emerging on the scene today. For example, new drugs such as MDMA, which acts as both a hallucinogen and a stimulant, require new treatment approaches, as does the unique stimulant methamphetamine. By having our "finger on the pulse" of these constantly changing drug trends, NIDA is in a position to use the power of scientific research and its application to avert emerging drug problems before they become national epidemics. Similar to the way epidemiologists work in teams with researchers from various disciplines to combat emerging infectious diseases, NIDA is expanding its portfolio to ensure a more rapid and community specific response to drug problems as they emerge. NIDA has a strong infrastructure already in place as an early-warning system, the Community Epidemiology Work Group, involving 21 sites around the country. In FY 2002 NIDA will develop a more integrated mechanism for linking research to a direct public health response by identifying potential public health hazards that are drug related, rapidly alerting the public and health communities, and stimulating new research to develop new treatments and antidotes.
Confronting the Methamphetamine and Club Drugs Crises. NIDA will continue to support research and dissemination of information that can increase our and the public's understanding of club drugs such as methamphetamine and MDMA. NIDA will place special emphasis on examining the medical consequences of club drugs at various developmental points (i.e. pre-adolescent, adolescent and college-aged). NIDA will also focus additional research and dissemination efforts at populations specifically known to be vulnerable to club drugs, such as gay and lesbian populations, and individuals in rural settings. A major new activity for NIDA in the club drug arena will be to more closely examine the health and developmental consequences of prenatal exposure to methamphetamine and its analogs such as MDMA. Use of these drugs is at very high levels in certain regions of the country. Anecdotal reports suggest that some pregnant women may have the perception that these drugs are not dangerous. Given NIDA's experience with prenatal cohort studies on the consequences of exposure to cocaine, NIDA is in an excellent position to study and attack this problem. NIDA is also increasing its efforts to alert the public and the health and science communities about the dangers of another emerging drug problem, gamma hydroxybutyrate (GHB). In addition to encouraging researchers to support studies that look more closely at the pharmacology and toxicology of GHB and GHB precursors, NIDA will work to better educate emergency room physicians and paramedics on how to better diagnose and treat patients who may present signs of GHB overdose.
Expanding Efforts at Reducing Nicotine Addiction. NIDA will continue to support a comprehensive research portfolio that focuses on nicotine, including the areas of basic, prevention, and treatment research as well as developing effective science-based educational outreach activities. NIDA will begin to formally work with the National Cancer Institute (NCI) and others to develop a more strategic process for developing new medications for nicotine dependence. NIDA will also continue to work with NCI and the Robert Wood Johnson Foundation to support the existing seven tobacco research Centers that have been established across the country. The Centers provide an exemplary model of how scientists with expertise in areas that include molecular biology, genetics, neuroscience, epidemiology, imaging, primary care, behavioral science, communications, health policy, economic, and marketing can work together to tackle a significant public health problem like nicotine addition.
Determining the Factors That Make Individuals Vulnerable to Addiction. Determining the genetic factors that make a person more or less susceptible to addiction is a critical area of research. In addition to searching for genes contributing to vulnerability, new genetic technologies are also helping us to elucidate the molecular and cellular mechanisms by which addiction develops over time and drug exposure. As an example, by manipulating the expression of specific genes, scientists have now produced "knockout" strains of mice for every major receptor involved in responsiveness to drug abuse. These new animal models are providing new insights into how drugs of abuse produce their complex effects. Identifying the genes that contribute to addiction vulnerability is only part of the story. Whether or not a person tries a drug and upon using drugs becomes addicted involves a complex interplay between a large number of environmental factors such as access to drugs, peer influences, and family involvement. NIDA will expand research in these areas. Moreover, research will focus on better understanding how the genetic factors involved in the addiction process interact with the environmental contributions to addiction.
The Next Generation of Prevention Research. NIDA has come a great distance over the past 25 years in developing and disseminating the fundamental principles of drug abuse prevention, but we are now ready to take our research to the next level. Prevention programs must be responsive to the special characteristics of different locales and settings, and to differences in the needs and responses of audiences that often vary in gender, ethnicity, and age. Given that communities want to adapt programs to meet specific needs, NIDA will support research that determines what aspects of content, delivery, client, and implementer account for effectiveness in prevention programs. NIDA will also support new research that determines ways for schools to adopt science-based programs and effectively incorporate those programs into their regular curriculum. Another new thrust for NIDA's prevention researchers will be to better understand factors that influence the selection, adaptation, organization, financing, and management of prevention programs at the community level.
Neuroimaging Across Childhood Development. Researchers have learned much from animal and human cohort studies about the detrimental effects that drugs can have on the brain. Though great progress has been made in this area, we still know relatively little about the neurodevelopmental consequences of prenatal drug exposure, or the effects of drugs of abuse on brain structure and function of children and adolescents who begin experimenting and abusing drugs while their brain is still developing. Drug addiction and neuroimaging researchers are now coming together to apply brain imaging techniques to assess and characterize the neurobiological consequences of drug exposure. Specifically, NIDA researchers will begin to use sophisticated brain imaging techniques to assess how drug exposure affects neurobiological, neurodevelopmental, and neurobehavioral processes while the child's brain is still developing. NIDA's existing multi-year cohort studies that continue to provide invaluable information about prenatal cocaine exposure provide an important resource opportunity for this endeavor as well.
Unearthing the Switch: Revealing the Mechanisms of and Transition to Addiction. NIDA supported research has significantly advanced understanding of the behavioral, biological, and molecular factors both underlying initial drug use and characterizing the state of being addicted. We know a great amount about both the biological and behavioral differences between a voluntary drug user and an addict. There is a tremendous gap, however, in understanding the mechanisms that underlie the actual transition from one state to the other Ð the change that occurs between being a voluntary drug user and an addict. This topic has been identified by the National Advisory Council on Drug Abuse as a major area requiring new, intensive, focused attention. NIDA is beginning to implement a multi-staged strategic effort to stimulate research to better understand these mechanisms.
NIDA Science Advances
Mapping the Cocaine High Versus the Natural Reward High. Researchers have known for many years that cocaine activates the brain's reward pathway in order to produce euphoria. Thus, research has focused on developing medications for cocaine addiction that can block cocaine's action on the reward pathway. But, this same pathway is activated by normal activities such as eating, drinking, and sex. If a medication were to prevent a person from experiencing feelings of reward from normal activities, it is unlikely that they would be willing to use it as a treatment for cocaine addiction. Researchers have now shown that cocaine activates a part of the reward pathway that is separate from those components of the pathway that are activated by food and water. This opens up the possibility of developing a medication for treating cocaine addiction that will interfere with cocaine's ability to produce reward without affecting other natural rewarding activities.
Craving for One Drug May Increase Cravings for Other Drugs. New findings suggest that craving for nicotine appears to increase craving for other illicit drugs among drug abusers who smoke tobacco. A recent study examined the relationship between craving for nicotine and other drugs, using a technique of "cue-induced" craving. Participants listened to recorded scripts describing scenes that were pleasant, unpleasant, or neutral. Some of the scripts mentioned smoking while others did not. The participants reported that their tobacco craving increased in intensity when listening to the smoking messages in the scripts. They also reported that craving for tobacco also elicited craving for the participant's other drug of choice. These results suggest that smokers in drug treatment programs may be less successful than nonsmokers in staying off drugs. Therefore, drug treatment programs should offer smoking cessation programs to individuals addicted to nicotine.
Successful Prevention Program Targets Steroid Abuse in High School Athletes. Anabolic steroid abuse is on the rise among 8th and 10th graders across the country. To counteract this trend, researchers have developed a unique, team-centered, gender-specific steroid abuse prevention program, Adolescents Training and Learning to Avoid Steroids (ATLAS). Evaluation of the ATLAS program in 31 Oregon high school football teams, showed that an athlete's intention to use and actual use of steroids was significantly lower among participants. At the year follow-up, illicit drug and alcohol use was reduced and other effects included fewer students drinking and driving, less use of dietary supplements, and improved nutritional behavior. A program designed for adolescent female athletes, patterned after ATLAS, is currently being tested.
Understanding Critical Gender and Ethnic Differences in Pathways to Drug Abuse Improves Prevention Efforts. Research shows that while there are similarities across all youth, ethnicity and gender play significant roles in both drug use and how and when an individual was offered drugs. Over 2,500 African American, Mexican American, and European American seventh grade students in Arizona were represented in a recent study that measured the relationship of ethnicity, gender, drug use, and resistance to drug offers and provided an in-depth understanding of how these young people encounter and respond to drugs in the real world. The findings showed that Mexican Americans received more offers, used more drugs, and were more likely to be offered drugs by peer family members and at parties. European Americans were more likely to receive drug offers from acquaintances, at friends' homes, and on the street. African Americans were more likely to receive drug offers in the park, from dating partners and parents, and were more likely to resist offers of drugs by using explanations. Gender significantly affected drug offers and types of offers. It was also noted that across all groups there was limited knowledge of how to resist drug offers. This research confirms that having the skills to resist drug offers is critical to the prevention of initial drug use.
New Behavioral Treatments for Marijuana Addiction. Marijuana is the most frequently used illegal drug in the United States. Despite the growing need for treatment, very few treatments are effective for addressing marijuana addiction. Researchers are now working to see if behavioral therapies that have been shown to be effective for other drugs of abuse work for individuals seeking treatment for marijuana addiction. In a recent study comparing three behavioral treatments, researchers found that individuals who received voucher incentives had higher marijuana abstinence rates after 14 weeks of treatment than those who did not receive vouchers. In the voucher-based incentive program, participants attended individual therapy sessions and could earn vouchers exchangeable for retail goods or services such as movie passes, sporting equipment, or vocational classes if their urine samples were determined to be drug free. When compared to either a motivational or a cognitive-behavioral therapy alone, the addition of a voucher-based incentive program to a behavioral intervention improved marijuana abstinence. These results add to the growing evidence that voucher-based incentive programs are an effective method for enhancing treatment outcomes.
What causes the pleasurable effects of cocaine? While the brain chemical dopamine has been found to play an important role in mediating the pleasurable effects of virtually all drugs of abuse, evidence is emerging that other brain chemical systems or neurotransmitters, such as serotonin, may also play a role in causing pleasure. Serotonin is known for its role in regulating body temperature, sleep, sensory perceptions and impulse control. Serotonin acts in the brain by attaching to molecules known as receptors. There are many receptors where serotonin can act. Using highly sophisticated bioengineering technology, scientists were able to produce a new strain of mice in which the gene that produces one type of serotonin receptor, called the 1B subtype, was missing or "knocked out." Researchers found that the knockout mice were more sensitive to the stimulant effects of cocaine and that the difference was related to increased dopamine in a particular brain area in these mice. This suggests that the serotonin 1B receptor subtype plays a critical role in regulating dopamine in the brain. Therefore, it appears that it is the interplay between these two neurotransmitter systems that contributes to the pleasurable effects of cocaine in these mice.
Smoking During Pregnancy May Increase the Risk of Behavioral Problems in Children. Approximately 800,000 children are born each year to women who smoke. Researchers found a relationship between smoking during pregnancy and behavioral problems in toddlers while studying two-year-olds of mothers who either smoked or did not smoke during pregnancy. This relationship was seen even after controlling for a number of psychosocial risk factors that have previously been shown to be associated with toddler's negative behaviors. In addition to maternal smoking, two other risk factors involving discipline and conflict in the mother-child relationship were identified. Three possible causes by which smoking may influence childhood behavioral problems were suggested: 1) smoking may narrow placental blood vessels affecting fetal nutrition and leading to problems with fetal brain development; 2) stressed mothers may tend to smoke and the stress itself may affect fetal brain development; 3) smoking affects the infant's health (e.g. low birth weight) which, in turn, affects the child's behavior when a toddler.
More Evidence that Methamphetamine Produces Long-term Damage to Human Brain Cells. Researchers have now provided direct cellular evidence that methamphetamine can cause long-lasting injury to the human brain that persists even after drug use has been discontinued. In a recent study, human subjects who had used methamphetamine for at least five years and had been abstinent an average of 4.25 months and for as long as 21 months were studied using a brain imaging technique that allows researchers to measure concentrations of certain biological markers that are associated with damage to neurons. One marker, N-acetylaspartate (NA), provides a measure of nerve cell viability. In abstinent, long-term methamphetamine abusers NA was reduced in several brain areas, indicating that neurons in these regions have been damaged. Two other markers, myoinositol and choline-containing compounds were elevated in the frontal cortex of the former methamphetamine abusers. These substances are present in glial cells, a special type of cell that surrounds and supports neurons. When neurons are damaged or die, glial cells proliferate, which results in an increase in myoinositol and choline-containing compounds. The increase in these substances along with the reductions of NA in the brains of former methamphetamine abusers indicates that methamphetamine can cause injury to neurons that is long-term and perhaps even permanent. This damage may explain persistent abnormal behaviors, such as violence, psychoses, and personality changes, which are often observed in former abusers months and even years after they have stopped using methamphetamine. These results also indicate that research efforts should focus not only on the development of treatments for addiction, but also on reversing any abuse-related damage.
Creating a Vaccine to Treat Nicotine Addiction. Nicotine addiction is at the root of one of this Nation's most serious public health problems, tobacco use. Using immunological techniques, researchers have developed a vaccine that prevents nicotine from reaching the brain. When injected in laboratory animals, the vaccine stimulates the immune system to produce antibodies that bind tightly to nicotine. The antibody-bound nicotine is too large to enter the brain, thereby preventing nicotine from producing its many effects. When nicotine was given to vaccinated rats, scientists found that the amount of nicotine reaching the brain was reduced by 64 percent and nicotine's ability to increase blood pressure and stimulate locomotor movement in rats was blocked.
Genetics May be Key to New Treatment for Nicotine Addiction. Researchers have found that individuals with a genetic deficiency in nicotine metabolism mediated by an enzyme in the liver (CYP2A6) are less likely to start smoking, and smoke less if they do start, than individuals with normal CYP2A6 activity. Building on this knowledge, scientists tested more than 200 medications to find compounds that decreased CYP2A6 activity. They found that methoxsalen, used to treat skin disorders, significantly reduced nicotine metabolism. To look at how methoxsalen affected cigarette smoking, the researchers conducted two studies. In one study, 17 regular smokers with normal CYP2A6 metabolism received methoxsalen or placebo in combination with oral nicotine replacement. The participants who took high doses of methsoxsalen reported far less desire to smoke and had nicotine levels in their blood roughly twice as high as those given placebo or low doses of methoxsalen. By decreasing nicotine metabolism, which keeps more nicotine in the blood, smokers needed fewer cigarettes to replace nicotine lost to metabolism. In a second study, participants received either methoxsalen or placebo in combination with nicotine or placebo and after a 60-minute period of abstinence were allowed to smoke at will for 90 minutes. Smokers who received methoxsalen plus nicotine smoked fewer cigarettes, had longer intervals between cigarettes, and took fewer puffs on each cigarette.
A Combination of Buprenorphine and Disulfiram Appears Effective In Treating Individuals Addicted to Heroin and Cocaine. More than 50 percent of individuals addicted to opiates, such as heroin, are also addicted to cocaine. The development of medications for treating these dual addictions is of the utmost importance. A recent study found that participants addicted to both opiates and cocaine who received a combination of disulfiram and buprenorphine abstained from cocaine use for longer periods of time than those who received only buprenorphine. These same participants also achieved three weeks of continuous cocaine abstinence sooner than those who received buprenorphine alone. No significant differences were found in the total weeks of opiate abstinence between the disulfiram/buprenorphine and the buprenorphine-only group. This research also reinforces previous studies that suggest administering disulfiram prior to cocaine inhalation may block the pleasurable and rewarding effects caused by an excessive release of dopamine in the brain after cocaine use. Instead of experiencing euphoria and other feelings of well being associated with cocaine use, an individual who has taken disulfiram before using cocaine will experience adverse reactions such as anxiety, dysphoria or paranoia. These results are promising for the potential effectiveness of disulfiram treatment for cocaine addiction in heroin addicts who are being treated with buprenorphine.
School-based Program Reduces Smoking Among Minority Girls. In order to stem the increase in smoking among minority girls, investigators tailored language, concept examples, and situations in a 15-session smoking prevention program (found previously successful for white youth) to 7th grade minority girls in 29 inner-city schools. The program focused on the development of social resistance skills and personal and social competence development. Researchers found that girls who participated in the smoking cessation program were less likely to initiate smoking if they had not previously smoked when compared to girls who did not participate in the intervention. Overall, participants had lower levels of intentions to smoke, more accurate perceptions of peer and adult smoking behaviors, better refusal skills, and reported fewer risk behaviors. Effective prevention programs need to teach students a variety of cognitive-behavioral skills to enhance assertiveness, resist advertising, manage anxiety, communicate effectively, and develop strong interpersonal skills.
Cocaine High Related to How and How Quickly the Drug Reaches the Brain. Epidemiological studies suggest that smoked and intravenous (IV) cocaine have greater abuse liability than intranasal cocaine. In some studies, subjects have also reported that smoked cocaine produces a greater "high" than cocaine taken intravenously. To examine the biological basis for these differences, researchers used positron emission tomography (PET) to compare the ability of cocaine to block dopamine transporters (DAT) in the brain. The DAT is the major target of cocaine and is believed to mediate the pleasurable effects of cocaine. Researchers found that equivalent amounts of cocaine given either intranasally, IV, or smoked, blocked similar numbers of DAT in each of the subjects. However, subjects who smoked cocaine reported a greater "high" than those who took cocaine intranasally. Those who received IV cocaine were intermediate in response, but most similar to those who smoked the drug. These findings suggest that the difference in reported "high" are not due to the level of DAT blockade. Rather, the cocaine "high" is more likely influenced by how fast the drug reaches the brain. Cocaine levels in the brain reached their peak amounts within two minutes for those who smoked, about three minutes for IV, and 15 minutes for the intranasal group, which parallels the reported "high" from the drug. Understanding how a drug produces its subjective effects will aid investigators in their efforts to develop more effective treatments for addiction.
Marijuana Ingredient May Promote Tumor Growth. Researchers found that THC (delta-9-tetrahydrocannabinol), the major psychoactive component of marijuana, may promote tumor growth. Intermittent doses of THC promoted the growth of tumors in mice injected with cancer cells by increasing the levels of specific cytokines (inter-cellular messengers) that signal the suppression of an immune response. The investigators also found that giving the mice a specific antagonist (neutralizer) of the cannabinoid receptor blocked the effect of THC. These findings suggest that THC promotes tumor growth by inhibiting the body's anti-tumor immune response. They also suggest that regular use of marijuana may increase the risk of respiratory tract cancer. More studies are needed to better understand the implications of these results.
New Insights into the Natural History of Hepatitis C Virus Infection in Injection Drug Users. A majority of injection drug users (IDUs) are infected with hepatitis C virus (HCV). In most people with HCV infection the virus persists, resulting in a chronic infection. After 10 to 30 years persons with persistent HCV infection may remain asymptomatic or may develop end stage liver disease (ESLD). Estimates of ESLD vary widely and there is little consensus on disease co-factors, such as HIV and hepatitis B infection, or predictors of disease progression. New data from a community-based cohort of IDUs demonstrate that the majority of those infected with HCV had persistent viral particles in the blood without clinically demonstrable liver disease. The incidence of ESLD was 3.1 cases per 1000 persons; persons 38 years and older had a fourfold increase in incidence of ESLD, and those drinking three or more drinks daily were 3.6 times more likely to have ESLD. The relative incidence of ESLD also increased with more frequent injection drug use. No increased risk of ESLD was observed for HIV-infected participants, for African Americans, or for those infected with hepatitis B, although clearance of HCV was less likely to occur among African-Americans and among IDUs infected with HIV. In this cohort of IDUs, only 1 of 1667 persons infected with HCV received therapy for HCV.
Male-female Differences in Drug Use Traced to Differences in Opportunity to Use. It has been theorized that males have higher rates of drug use than females because males have a greater chance of progressing from initial opportunity to use a drug to actual drug use. In assessing data from 131,226 respondents to the 1979 to 1994 National Household Survey on Drug Abuse, researchers found that males age 12 and older had more initial opportunities to use drugs than females of the same age. But the data showed that once an opportunity had occurred few male-female differences existed in the probability of making a transition into drug use. These results support the theory that males have higher rates of drug use because they have a greater number of initial opportunities to use drugs, not a higher probability of progressing to actual drug use. Thus, sex differences emerge in the earliest stages of drug involvement.
Treating Cocaine Addiction with an Ancient Chinese Therapy. Cocaine addiction is a significant public health problem, for which there are no treatment medications currently approved. Researchers are investigating the possibility of using acupuncture as a treatment for cocaine addiction. Eighty-two patients addicted to cocaine participated in the study. They were assigned to one of three groups: treatment with auricular acupuncture; a control condition where a needle was inserted into the ear where it likely wouldn't generate an effect; or a no-needle relaxation control where the patients viewed relaxation videos. Among the patients who completed the study, more than half of the acupuncture patients tested free of cocaine the last week of treatment compared to 23.5 percent of the control group and 9.1 percent of the relaxation group. The patients who received acupuncture also had longer periods of sustained abstinence compared to the other participants. Acupuncture when used in combination with other therapies may be an innovative, and effective way to treat cocaine addiction.
Attention Deficit Hyperactivity Disorder and Substance Abuse. There is continued concern but very little data regarding whether or not treating children with ADHD with stimulants, such as Ritalin, can lead to later drug abuse. A new study addressing this issue showed that boys with ADHD who received treatment were significantly less likely to abuse drugs and alcohol when older. Among study participants, the research showed that 75 percent of the non-medicated ADHD boys had at least one substance use disorder compared to 25 percent of the medicated ADHD boys and 18 percent of the boys without ADHD. These results indicate that accurately diagnosing and properly treating ADHD may help prevent emergence of substance abuse in these at-risk individuals.
Animals Will Self-administer THC, Marijuana's Active Component. This seminal research finally confirms that marijuana has potential for abuse that is at least as strong as that of cocaine and it shares important characteristics with other addicting substances. Researchers have for the first time reliably shown that monkeys will self-administer doses of THC comparable to the amount inhaled by humans while smoking marijuana. Importantly, researchers showed that the animals took THC with the same intensity as a comparison group of animals administering cocaine. Prior to the study, scientists established self-administration behavior in a group of squirrel monkeys that received intravenous injections of cocaine after pressing a lever 10 times for each injection. At the start of the study, the researchers replaced the cocaine with saline solution, which halted self-administration. Then, researchers substituted the saline for THC in a solution that would rapidly enter the brain in a manner similar to the rapid way in which THC enters the brain when marijuana is smoked. The monkeys resumed self-administration and worked to obtain, on average, 30 injections per one-hour session. Giving the monkeys a compound that inhibited THC from binding to its receptors in the brain caused the animals to stop taking THC, yet the same compound given to a control group of monkeys administering cocaine had no effect. Thus, the researchers confirmed that the compound negated the effects of THC but did not alter self-administration behavior more generally.
Adolescent Cigarette Smoking May Negatively Affect a Teen's Emotional Health. Cigarette smoking among youth may be even more harmful than was previously thought. A community-based longitudinal study of almost 700 youths showed for the first time that chronic cigarette smoking during adolescence may contribute to the onset of certain anxiety disorders. Diagnostic assessments and interviews were conducted among the participants and their mothers at various stages of development. Adolescents who smoked 20 cigarettes or more per day were found to be at increased risk for agoraphobia, generalized anxiety disorder, or panic disorder during early adulthood. This association held true even after the researchers controlled for age, gender, difficult childhood temperament, parental smoking, parental education, parental psychopathology, alcohol and drug use, and anxiety and depressive disorders during adolescence. Cigarette smoking was not, however, found to be associated with risk for obsessive-compulsive disorder or social anxiety disorder. The researchers also found no evidence to suggest that adolescents with anxiety disorders are at any increased risk for chronic smoking during early adulthood. Providing adolescents with information that chronic cigarette smoking may increase risk for the onset of anxiety disorders may increase the effectiveness of prevention efforts.
Using "Rational" Drug Design Techniques to Search for Possible New Anti-cocaine Medications. Using new computational methods researchers have been able to screen large libraries of chemical compounds and identify five possible leads for the development of new anti-cocaine medications. Researchers studying the dopamine transporter and its role in cocaine's potential for abuse, have developed a three-dimensional method, known as 3D Quantitative Structure Activity Relationship (QSAR), for creating models of the structure of this transporter and the ligands, or compounds, that bind to it. This approach to searching for potential drug compounds by looking at a model of the biological target and its activity is known as "rational" drug design. The researchers have now enhanced their 3D-QSAR method by incorporating a two-dimensional algorithm which reduces the need to create so many possible conformations of the target structure and reduces mathematical complexity. Thus, 3D-2D QSAR is better suited for searching databases of compounds for possible drug leads. This combined method was used to search the National Cancer Institute's database of possible drug compounds leading to the identification of five compounds with potential for treating cocaine addiction. Furthermore, this technology could be applied to the search for possible medications that target other transporter or receptor systems involved in drug abuse.
Natural Compound May Offer New Treatment for Chronic Pain. Chronic pain associated with nerve inflammation, nerve injury (neuropathy), and spinal cord injury can be difficult to treat. Generally opiates, such as morphine, are ineffective at relieving these types of pain. Consequently, much pain research is focused on gaining a greater understanding of mechanisms underlying these types of pain and developing new effective treatments that can reduce or eliminate chronic pain. In this study, animal models of various types of pain were used to test whether agmatine, a newly discovered substance that is naturally present in brain and spinal cord, could reduce or eliminate pain from nerve inflammation, nerve injury, and spinal cord injury. Agmatine was found to reverse pain caused by nerve inflammation or nerve injury. It was also found to reduce changes that occur in the spinal cord as a result of injury. These results suggest that the agmatine that is present in brain and spinal cord may play a role in pain control. Importantly, they also suggest that agmatine may be highly effective as a treatment for some types of pain that are difficult and sometimes impossible to treat with currently available pain medications. The results also suggest that agmatine may help reduce damage to the nervous system caused by spinal cord injury. This could not only result in a dramatic reduction in pain associated with spinal cord injury, but also in a reduction the extent of the injury and therefore in functional impairment.
Ecstasy Tablets May Contain Other Drugs that Can Cause Serious Adverse Reactions. Ecstasy use by our Nation's youth has been increasing. Until now little research has been conducted on the actual content of ecstasy tablets sold in the United States. A recent analysis of 107 ecstasy tablets received anonymously from across the country, researchers found that although most contained some MDMA or one of its derivatives, nearly 30 percent of the tablets tested contained drugs other than MDMA or no drugs at all. The other drug most commonly identified was dextromethorphan (DXM), an over-the-counter cough suppressant, which was found in 23 pills. Additional drugs identified included caffeine, ephedrine, pseudoephedrine, acetaminophen, and salicylates. The amounts of dextromethorphan (DXM) found in the tablets were higher than the usual doses of the drug found in cough syrups. Thus, ecstasy users, who often take multiple tablets, could be ingesting high doses of DXM alone or with MDMA. At doses of over 300 milligrams, DXM can cause lethargy or extreme excitability, increased heart rate, and uncoordinated muscle action, as well as psychosis similar to that seen with PCP use. Thus, high doses of DXM may contribute to some of the serious adverse reactions attributed to MDMA abuse. In addition, MDMA's presence may compound reactions to DXM because MDMA inhibits the action of an enzyme (cytochrome P450) that plays a critical role in processing and removing drugs like DXM from the body. Physicians and other health care providers need to be alerted to the fact that a patient that appears to be having an adverse reaction to MDMA but does not test positive for the drug may be reacting to DXM.
Story of Discovery - Bringing a New Medication to Market: Shifting Treatment From Clinics to Doctors' Offices
Although it may seem like new medications for treating diseases come on the market every day, this is clearly not the case for drug addiction, where new medications appear on the market or are approved for use much less frequently. For example, the last anti-heroin addiction medication to become available was LAAM which was approved for the management of opiate dependence in 1993. Unfortunately like the only other agonist medication approved for treating opiate addiction, methadone, both medications are only available in a limited number of clinics across the country. There are strict state and federal regulations that control the use of these medications, frequently making it difficult for the estimated 900,000 individuals in need of opiate treatment to receive it. The good news is, there are two new medications that once approved by FDA, will be available to qualified physicians across the country to prescribe in their own office settings. Having safe and effective alternative pharmacotherapies for treating opiate addiction that can be dispensed in doctors' offices will dramatically increase access to drug addiction treatment.
The two new medications that NIDA has just brought to fruition are buprenorphine (Subutex) and buprenorphine/naloxone (Suboxone). Both products contain the active ingredient buprenorphine, a partial agonist that functions on the same brain receptors as heroin, but does not produce the same high, dependence, or withdrawal syndrome. Buprenorphine actually prevents heroin from binding to opiate receptors, thus blocking its pleasurable effects. Buprenophine also blocks withdrawal discomfort by keeping the receptors occupied. It is long-lasting, less likely to cause respiratory depression, well-tolerated by addicts and when combined with naloxone, has very limited diversion potential.
Bringing these buprenorphine products to their current state of development was not an easy task. In fact, the search for a medication like buprenorphine has been underway since at least 1929. It was in the 1920s when the United States began to seriously recognize that Americans were abusing and becoming addicted to opiates, especially as a way to treat pain. A number of formal projects were initiated to replace opiates with medications that could treat pain, but did not have addictive properties. Thousands of synthetic opioids were produced and tested. As these formal efforts to identify opioids of low abuse liability waned during the 1970s, the last major compound to be tested as a result of this effort was buprenorphine.
Buprenorphine was first synthesized in 1969 in England by Dr. John Lewis of Reckitt and Colman Products and subsequently developed as an analgesic. It was initially being looked at purely for its abuse liability potential, but was discovered to have properties that could be used therapeutically. It was first marketed in the United Kingdom in 1978 for injection and in 1981 and 1982 as an oral tablet. It is being marketed in over 40 countries today as an analgesic. It has been marketed in the United States since 1985, but only in its injectable form. In the mid 1970s researchers at the Addiction Research Center in Lexington, Kentucky (NIDA's intramural program at that time) began to take an interest in buprenorphine as a medication that might work for treating opiate addiction. In 1978, Dr. Donald Jasinski and colleagues in a landmark clinical study showed that buprenorphine can in fact block the euphoria produced by opiates (Archives of General Psychiatry 35:501-516). Other researchers were also reporting that daily administration of buprenorphine decreased heroin self-administration in opiate abusers.
Studies on buprenorphine continued throughout the 1980s and 90s. Congress, recognizing the need to stimulate the availability of addiction medications, passed several pieces of precursor legislation during the 1970s and 80s indicating its intention that NIDA initiate and promote research into the creation, development and testing of pharmacological substances for treatment of addiction. NIDA administratively created a Medications Development Division to focus on this effort in 1990. By 1992, Congress passed the "ADAMHA Reorganization Act" (P.L. 102-321) which statutorily established the Medications Development Program in NIDA. By this time, the new medications program included a number of major studies to document buprenorphine's safety and efficacy in the opiate-abusing population. Researchers had accumulated data from more than a half-dozen controlled clinical trials involving over 1000 patients, using outcome measures of illicit opiate use and retention in treatment to substantiate buprenorphine's clinical safety and efficacy. With the bulk of the research completed, NIDA established a Cooperative Research and Development Agreement in 1994 with the original developers of the medication, Reckitt and Colman Pharmaceuticals, Inc. This was a team effort to bring this drug to a marketable status for treatment of opiate addiction in the United States. In 1999, Reckitt submitted all of the study data to the FDA in support of a new drug application (NDA) for buprenorphine in the treatment of opiate dependence. Once final approval comes from the FDA, these medications will become the first of their kind to be dispensed more like other commonly prescribed medications, such as those used to treat diabetes or hypertension.
On October 17, 2000, an historic day for addiction treatment, a bill was signed that allows qualified physicians to prescribe certain anti-addiction medications in an office setting, including buprenorphine and buprenorphine/naloxone (after approval by the FDA). Although the Drug Addiction Treatment Act is a small provision in the Children's Health Act (HR 4365), it is a gigantic leap for science and for those in need of addiction treatment. This treatment provision amends the Controlled Substances Act, which placed strict requirements on practitioners dispensing narcotics for treating addiction. For the first time, the disease of addiction will be put on an equal footing with other chronic diseases. Buprenorphine and buprenorphine/naloxone products are expected to increase the amount of treatment capacity available and expand the range of treatment options that can be used by physicians.
Story of Discovery - Glimpses into the Working Brain: The Development of Neuroimaging Tools to Advance Our Understanding of Addiction
There is a scene in a Sherlock Holmes story where Detective Holmes and his archvillain first meet. After looking intently at Holmes' forehead, the villain remarks, "You have less frontal development than I should have expected." This statement is an allusion to an early study of the brain called "Phrenology." Developed in the early 1790s by Franz-Joseph Gall, Phrenology, hypothesized that an individual's skull directly reflected underlying brain structure and thus function. The shape of one's brain was also thought to tell you about that person's character. Phrenology reflected the state of understanding of the brain at that time Ð that is, using the gross shape of the skull to provide insights into both the brain and the person. Phrenology was discredited by scientific research but led to some of the first notions of how the brain and behavior were related.
Our understanding of brain function since the 18th century has advanced tremendously, although amazingly, the underpinnings of today's modern neuroimaging technologies were built on findings from that era. In 1890, the noted psychologist, William James, wrote that blood flow to the brain was related to brain function. Additionally, the seminal experimental work by the British Nobel Prize winning physiologist, Sir Charles Sherrington, demonstrated that blood flow increases in parallel with neuronal activity. These advances came about at the same time that scientists were gaining a basic understanding of how information is processed in the brain. As the world began to learn about how complex the brain actually is with its neurons, neurotransmitters and receptors, little did we realize that soon we would actually be able to see this complex structure that guides all of our thoughts, feelings, and behaviors. It was the recognition of the link between neural activity and blood flow that formed the empirical foundation for today's brain imaging methods, such as positron emission tomography and functional magnetic resonance imaging.
The hardware behind these brain imaging technologies dates back over 100 years as well. In 1895, the German physicist, Wilhelm Conrad Roentgen, discovered the X-ray, which subsequently gave medical and research science a way of visualizing the living brain through the skull. This discovery won him the first Nobel prize for physics in 1901. It was not until 1972 when the X-ray technology was further refined by Sir Godfrey Hounsfield. Hounsfield introduced the world to X-ray computed tomography (CT). The CT combines X-rays and computer imaging to create images that are more detailed than the standard X-ray. Although this advance rapidly began to change how we looked at the human brain, it still only produced anatomical pictures or essentially "snapshots" that did little to characterize what we now know as the dynamic process of brain function. The stage, however, was now set to develop tools that could inform practitioners and researchers as to what was happening in the brain, especially in response to substances of abuse.
By the mid 1970s, positron emission tomography (PET) was developed and utilized to study various brain-based diseases and disorders, including addiction. PET was used to show the dynamics of blood flow, to measure the metabolism of glucose (the fuel for brain cell function), as well as to characterize brain receptor systems. In the early 1980s, magnetic resonance imaging (MRI) began to be used in medicine. This imaging technique takes advantage of the magnetic properties of blood itself to reveal blood flow and activity within the brain. Both PET and MRI methods have been used by researchers to provide new insights into how brain function directs human behavior. The earliest studies using neuroimaging in humans to elucidate the biology of addiction was thought to be conducted in the mid- to late-1980s. Up to that point, much of what was known of the biology of addiction came from animal studies. By applying these methods to addiction, we were able to gain powerful, new insights into how exposure to drugs (of abuse) changed brain structure and function. It was possible to determine which brain circuits, pathways and systems were most involved and affected by abused drugs. The delineation between the acute and chronic effects of drug exposure was now possible. Most importantly, imaging clearly helped to establish that the brain could be structurally and functionally changed by drugs of abuse. We now had the proof to definitively show that addiction is, in fact, a brain disease.
Specifically, in the mid-1980s a turning point in our understanding of drug-brain-behavior interaction in humans came with two studies using PET. This work was extremely important because neuroimaging clearly illustrated the effects of a drug of abuse in the brain of a living person. Dr. Nora Volkow and her colleagues at Brookhaven National Laboratory revealed the path taken by cocaine and where in the brain it became localized, as well as the time course of its presence in and clearance from the human brain. She literally showed us a minute by minute sequence of a person's brain on cocaine. Importantly, this time course of cocaine's actions in the brain was directly correlated with its pleasurable experience (Synapse 1989; 4(4):371-377). Dr. Edythe London and her colleagues performed a complementary study showing the effects of cocaine on the brain functioning (Synapse 1992; 11:184-190). For the first time, these studies revealed how the brain changed when cocaine was administered. Later studies by Dr. Hans Brieter and colleagues took advantage of the rapid imaging capabilities of functional MRI to show in a time-lapsed sequence, virtually second by second, just how quickly cocaine affects a particular area of the brain. Neuroimaging studies of addiction are now being applied to show us what if any long-term effects drugs may have on the brain. This approach has been particularly useful as we attempt to understand the long-term effects on the brain of drugs being used increasingly across the country such as methamphetamine and MDMA (Ecstasy). Through the use of these modern imaging techniques, we now have direct evidence in humans to support the voluminous animal literature showing a decrease in a structural component of serotonergic or brain 5-HT neurons in human MDMA users. Additionally, we are now beginning to see signs that MDMA has residual effects as well Ð it can impair one's cognitive abilities. Further, these techniques can also be used to help us understand if the brain can fully recover from drug abuse and addiction. Are drug-induced brain changes permanent? Can abstinence or treatment allow the brain to change back to more normal structural and functional states? These are some of the questions we hope to be able to answer through modern science.
Thanks to one advance after another, neurobiologists can peer into the living human brain and produce images that shed new light on brain function especially as it related to drugs of abuse. The future holds even more promising results and new insights that will unlock many of the remaining mysteries of the brain.
NIDA Budget Policy
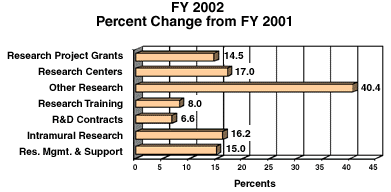
The Fiscal Year 2002 budget request for the NIDA is $907,369,000, including AIDS, an increase of $126,394,000 and 16.2 percent over the FY 2001 level, and $211,077,000 and 30.3 percent over FY 2000.
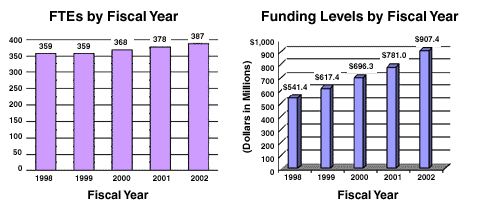
A five year history of FTEs and Funding Levels for NIDA are shown in the graphs below:
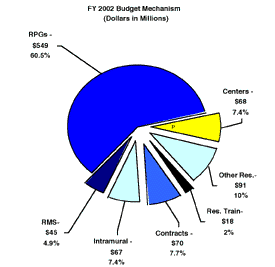
One of NIH's highest priorities is the funding of medical research through research project grants (RPGs). Support for RPGs allows NIH to sustain the scientific momentum of investigator-initiated research while providing new research opportunities. The Fiscal Year 2002 request provides average cost increases for competing RPGs equal to the Biomedical Research and Development Price Index (BRDPI), estimated at 4.3 percent. Noncompeting RPGs will receive increases of 3 percent on average for recurring direct costs. In FY 2002, total RPGs funded will be 1,462 awards, an increase of 124 awards over the FY 2001 Estimate, the highest annual total ever awarded.
Promises for advancement in medical research are dependent on a continuing supply of new investigators with new ideas. In the Fiscal Year 2002 request, NIDA will support 430 pre- and postdoctoral trainees in full-time training positions. An increase of 10 percent over Fiscal Year 2001 levels is provided for stipends and training-related expenses (e.g., health insurance, research supplies and equipment, and travel to scientific meetings).
The Fiscal Year 2002 request includes funding for 34 research centers, 265 other research grants, including 205 new clinical career awards, and 126 R&D contracts. The R&D contracts mechanism also includes support for 17 contracts for the Extramural Clinical and Pediatric Loan Repayment Programs.
The FY 2002 Request for research management and support (RMS) is an increase of 15% above the FY 2001 level. Support for the increases in NIDA's extramural budget will require staff to coordinate front-line collaborations of researchers with service providers at the local levels in communities and schools in implementing new intervention programs, as well as educators and patients themselves seeking expanded, more directly applicable information. Enhanced RMS resources will also help to ensure active input from and collaborations with racial/ethnic minority populations to better understand the incidence of drug abuse in racial/ethnic groups, expand the community and racial infrastructure for conducting research, and improve prevention and treatment programs for groups at highest risk for addiction and its medical consequences. In addition, NIDA is initiating or expanding a number of major nationwide clinical and intervention programs. Included will be a new cross-agency Criminal Justice Treatment Initiative in response to increasing interest in this area. NIDA will encourage researchers to conduct rigorous scientific study to better understand the types of treatment programs that are now being used at various points while addicted offenders are under criminal justice control, identify the most successful or model programs, and from that information develop a set of best practice principles about duration, setting, and training to help frame a more effective system of treatment for addicted criminal offenders. At the same time, NIDA will stimulate research designed to foster the development of even more effective science-based approaches to treatment for this unique patient population Moreover, the National Drug Abuse Treatment Clinical Trials Network (CTN) will be expanded in FY 2002. Each of these is extremely staff intensive.
The mechanism distribution by dollars and percent change are displayed below: