Congressional Justification for National Institute on Drug Abuse
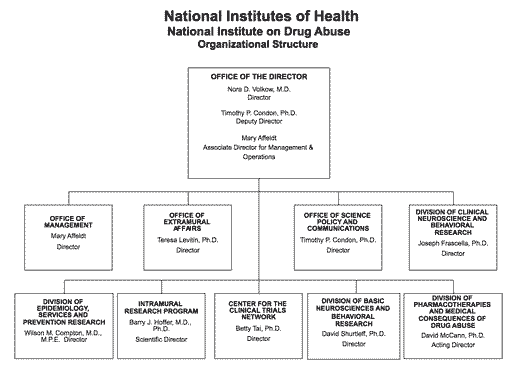
Organizational Chart
Appropriations Language
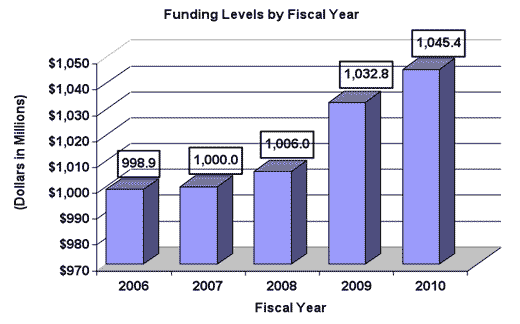
For carrying out section 301 and title IV of the Public Health Services Act with respect to drug abuse [$1,032,759,000] $1,045,384,000 (Department of Health and Human Services Appropriation Act, 2009)
| Source of Funding | FY 2008 Actual | FY 2009 Estimate | FY 2010 PB |
|---|---|---|---|
| Appropriation | $1,018,493,000 | $1,032,759,000 | $1,045,384,000 |
| Rescission | -17,793,000 | 0 | 0 |
| Supplemental | 5,322,000 | 0 | 0 |
| Subtotal, adjusted appropriation | 1,006,022,000 | 1,032,759,000 | 1,045,384,000 |
| Real transfer under Director's one-percent transfer authority (GEI) | 1,273,000 | 0 | 0 |
| Comparative transfer under Director's one-percent transfer authority (GEI) | -1,273,000 | 0 | 0 |
| Subtotal, adjusted balance authority | 1,006,022,000 | 1,032,759,000 | 1,045,384,000 |
| Unobligated balance, start of year | 0 | 0 | 0 |
| Unobligated balance, end of year | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | 1,006,022,000 | 1,032,759,000 | 1,045,384,000 |
| Unobligated balance lapsing | 0 | 0 | 0 |
| Total obligations | 1,006,022,000 | 1,032,759,000 | 1,045,384,000 |
Excludes the following amounts for reimbursable activities carried out by this account: FY 2008 = $4,024,000 FY 2009 Estimate = $4,451,000 FY 2010 Estimate = $4,477,000. Excludes $179,000 Actual in FY 2008; Estimate $179,000 in FY 2009 and Estimate $179,000 in FY 2010 for royalties.
| FY 2008 Actual |
FY 2008 Comparable |
FY 2009 Estimate |
FY 2010 PB |
Change | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Extramural Research: | FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount |
| Detail: | ||||||||||
| Basic and Clinical Neuroscience and Behavorial Research | $472,687 | $472,549 | $485,386 | $491,018 | $5,632 | |||||
| Epidemiology, Services and Prevention Research | 237,448 | 236,882 | 243,317 | 246,140 | 2,823 | |||||
| Pharmacotherapies and Medical Consequences | 114,346 | 114,087 | 117,186 | 118,546 | 1,360 | |||||
| Clinical Trials Nework | 40,393 | 40,393 | 41,490 | 41,972 | 482 | |||||
| Subtotal, Extramural | 864,874 | 863,911 | 887,379 | 897,676 | 10,297 | |||||
| Intramural research | 123 | 84,489 | 123 | 84,332 | 122 | 86,272 | 122 | 87,566 | 0 | 1,294 |
| Res. management & support | 253 | 57,932 | 253 | 57,779 | 262 | 59,108 | 270 | 60,142 | 8 | 1,034 |
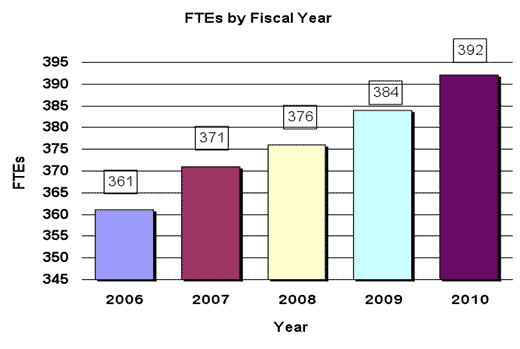
| TOTAL | 376 | 1,007,295 | 376 | 1,006,022 | 384 | 1,032,759 | 392 | 1,045,384 | 8 | 12,625 |
|
Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research |
||||||||||
Major Changes in Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2010 budget request for NIDA, which is $12.625 million greater than the FY 2009 Estimate, for a total of $1.045 billion.
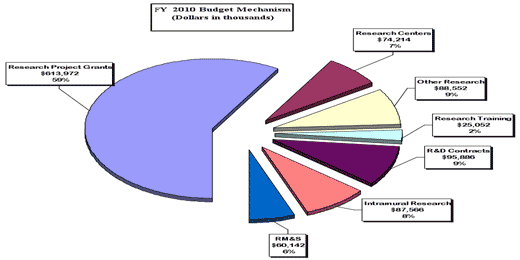
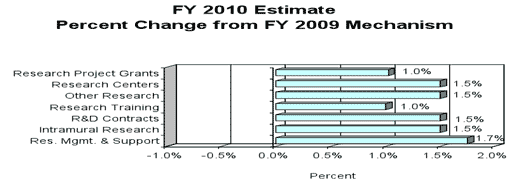
Research Project Grants (+$6.226 million; total $614.972 million): The NIH budget policy for Research Project Grants (RPGs) in FY 2010 is to provide inflationary 2 percent increases in noncompeting awards and a 2 percent increase in the average cost of competing RPGs. NIDA will continue to support new investigators while maintaining an adequate number of competing RPGs. NIDA will support a total of 1,415 Research Project Grant (RPG) awards in FY 2010. Non competing RPGs will decrease by 42 awards and decrease by $4.5 million. New and Competing RPGs will increase by 29 awards and increase by $12.6 million.
Intramural Research (+$1.294 million; total $87.566 million): Intramural Research will receive an increase of 1.5 percent to help cover the cost of pay and other increases. NIDA will work to identify areas of potential savings within the Intramural Research Program that will allow the institute to continue to achieve its program goals of performing cutting edge research within a multidisciplinary framework.
Research Management and Support (+$1.034 million; total $60.142 million): The NIDA oversees almost 1,900 research grants and more than 500 full-time training positions and over 200 research and development contracts. The 1.7 percent increase will partially be used to cover the expenses associated with pay raises and other inflationary cost increases necessary to provide for the effective administrative, planning and evaluation, public information and communications, and scientific leadership of the institute.
- Summary of Changes Table
-
Overall
- FY 2009 estimate - $1,032,759,000
- FY 2010 Estimated Budget Authority -1,045,384,000
- Net Change - 12,625,000
Summary of Changes Table Changes 2009 Current Estimate Base Change from Base Actual Number Budget Authority Number Budget Authority A. Built-in:
1. Intramural research:a. Annualization of January 2009 pay increase $19,963,000 $239,000 b. January FY 2010 pay increase 19,963,000 299,000 c. Zero less days of pay 19,963,000 0 d. Payment for centrally furnished services 8,446,000 169,000 e. Increased cost of laboratory supplies, materials, and other expenses 57,863,000 939,000 Subtotal
2. Research management and support:1,646,000 a. Annualization of January 2009 pay increase $33,404,000 $399,000 b. January FY 2010 pay increase 33,404,000 501,000 c. Zero less days of pay 33,404,000 0 d. Payment for centrally furnished services 6,089,000 122,000 e. Increased cost of laboratory supplies, materials, and other expenses 19,615,000 327,000 Subtotal
Subtotal, Built-in1,349,000
2,995,000B. Program:
1. Research project grants:a. Noncompeting 1058 $455,948,000 (42) ($7,420,000) b. Competing 370 133,646,000 29 13,365,000 c. SBIR/STTR 60 18,152,000 2 281,000 Total 1488 607,746,000 (11) 6,226,000 2. Research centers 47 73,117,000 0 1,097,000 3. Other research 339 87,243,000 3 1,309,000 4. Research training 564 24,804,000 5 248,000 5. Research and development contracts 203 94,469,000 3 1,417,000 Subtotal, extramural 10,297,000 FTEs FTEs 6. Intramural research 122 86,272,000 0 (352,000) 7. Research management and support 262 59,108,000 8 (315,000) 8. Construction 0 0 9. Buildings and Facilities 0 0 Subtotal, program 1,032,759,000 9,630,000 Total Changes 384 8 12,625,000
Budget Graphs
History of Budget Authority and FTE's
Distribution by Mechanism
Change in Selected Mechanisms
Justification of Budget Request
Authorizing Legislation: Section 301 and title IV of the Public Health Service Act, as amended.
| FY 2008 Appropriation | FY 2009 Omnibus | FY 2009 Recovery Act | FY 2010 President's Budget | FY 2010 +/- 2009 Omnibus | |
|---|---|---|---|---|---|
| BA | $1,006,022,000 | $1,032,759,000 | $261,156,000 | $1,045,384,000 | +$12,625,000 |
| FTE | 376 | 384 | 392 | +8 |
This document provides justification for the Fiscal Year (FY) 2010 activities of the National Institute on Drug Abuse (NIDA), including HIV/AIDS activities. Details of the FY 2010 HIV/AIDS activities are in the "Office of AIDS Research (OAR)" Section of the Overview. Details on the Common Fund are located in the Overview, Volume One. Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Director's Overview
Scientific advances continue to improve our understanding of drug addiction as a disease of the brain. New knowledge is revealing an increasingly detailed picture of the molecular, cellular, and circuit level changes that can lead to compulsive drug use and addiction. The need for this knowledge is urgent, as drug abuse and addiction continue to cause immeasurable morbidity and mortality, and to cost our society more than half a trillion dollars annually.
The National Institute on Drug Abuse's (NIDA's) support of science has generated knowledge used to develop prevention interventions that have contributed to the declines in both licit and illicit drug use, particularly among our Nation's youth. NIDA's 2008 Monitoring the Future (MTF) Survey reports a 25-percent decline in illicit drug use among 8th, 10th, and 12th graders combined, between 2001 and 2008, with cigarette smoking at its lowest rate since the survey began in 1975. Still, drug abuse and addiction remain highly prevalent, and changes in the drug culture require vigilance to curtail their spread. Particularly worrisome is the non-medical use of prescription and over-the-counter medications, most severe for opiate analgesics (e.g, hydrocodone and oxycodone). Abuse of these drugs rose throughout the 1990s and has remained stubbornly steady among adolescents during recent years, surpassed only by marijuana in rates of abuse.1
Another overarching challenge is how to deliver treatment to those who need it, most of whom go without. Thus, we remain committed to translating research for use in community settings through support of effectiveness research, including our National Drug Abuse Treatment Clinical Trials Network (CTN) and Criminal Justice-Drug Abuse Treatment Studies (CJ-DATS), and through educational outreach to judges, physicians, treatment providers, single state authorities, and other stakeholders.
New Tools, New Opportunities
We now have more sensitive and less costly tools to identify the genetic variations that increase vulnerability for addiction and related health consequences, including cancer. Cigarette smoking, the most preventable cause of cancer, is a primary target of NIDA's research portfolio. Recent genome-wide association studies of nicotine addiction, for example, have pointed to previously unsuspected genes whose products may be involved in the addiction process and in the susceptibility to smoking-related diseases, such as lung cancer and peripheral arterial disease (see "Portrait of a Program: Genetics and Addiction"). Knowledge gleaned from genetics research on addiction and its medical consequences, such as cancer, will not only help identify predictors of disease vulnerability, but will help optimize treatments to improve the public health. In support of the Presidential Initiative, NIDA will increase its cancer research funding by 4.4 percent to $10.950 million.
To complement these efforts, NIDA is investing in the rapidly evolving field of epigenetics, which focuses on the lasting modifications to DNA structure and function from exposure to various stimuli (e.g., parenting quality, stress, diet, drugs, etc.). A better understanding of how to exploit epigenetic changes to reduce vulnerability or counter the effects of abuse and addiction could result in unprecedented opportunities to enhance addiction treatment efficacy. While these genetic and epigenetic tools will greatly expand our ability to predict addiction risk and treatment success, new research designed to develop a comprehensive panel of addiction biomarkers could produce an addiction "signature" that could be used to assess chronic exposure to drugs and to monitor the effects of a given course of therapy.
Other emerging opportunities are found in interventions using web- and computer-based technologies, which have produced positive outcomes for drug abuse and HIV risk behaviors. NIDA-supported research will continue to investigate how such interactive technology can be integrated into the addiction treatment system to improve its effectiveness and bring about more widespread adoption of evidence-based approaches.
Partnering with Physicians and the Health System
Physicians can be the frontline for identifying patients who are abusing drugs that may put their health at risk even before problems arise. Thus, NIDA is set to disseminate a web-based "toolkit" to help physicians screen their patients for abuse of both licit and illicit substances, including prescription drugs, so these patients can be referred to treatment. NIDA also continues to support research on the impact and cost effectiveness of physician screening, brief intervention, and referral to treatment (SBIRT) for substance abuse.
The development of new medications may also serve to engage physicians more fully. Indeed, medications development is a crucial component of NIDA's research portfolio, particularly as efforts to engage the private sector have been met with limited success because of perceived financial disincentives and addiction-related stigma. Advances in our understanding of addiction neurobiology are revealing new molecules and structures that could be targets for addiction therapies. These include medications aimed at methamphetamine and cannabis addiction; vaccines for cocaine, nicotine, heroin, and methamphetamine addiction; pain medications without addictive liability; and new entities based upon recently identified candidate receptors or receptor combinations.
New Strategies in the Fight against HIV/AIDS and Drug Abuse
Drug use and HIV are inextricably linked - intravenous use is responsible for roughly one-third of HIV infections in this country since the epidemic began, and prevalence rates among non-injection drug users can be just as high. NIDA therefore supports research aimed at reducing HIV transmission, including finding innovative ways to incorporate HIV education, testing, counseling, and treatment referral in community settings, and overcoming barriers, such as stigma and inadequate access to HIV and drug abuse treatment. To attract innovative scientists, NIDA created an Avant-Garde award for high-impact research likely to foster groundbreaking approaches to prevent and treat HIV/AIDS in drug abusers. NIDA also continues to target HIV/AIDS-related health disparities and integrate HIV/AIDS initiatives worldwide (see "Portrait of a Program: NIDA's International HIV/AIDS-Drug Abuse Efforts").
Conclusion
NIDA's portfolio reflects a comprehensive approach aimed at developing knowledge that can transform the way we prevent and treat drug abuse and addiction, and that can be translated into the clinic and the community.
(1Johnston, L. D., O'Malley, P. M., Bachman, J. G., & Schulenberg, J. E. (2008). Monitoring the Future national survey results on drug use, 1975-2007. Volume I: Secondary school students (NIH Publication No. 08-6418A). Bethesda, MD: National Institute on Drug Abuse, 707 pp.)
Overall Budget Policy
NIDA will continue to support new investigators and to maintain an adequate number of competing RPGs. NIDA is providing a 2 percent inflationary increase for both non-competing and competing grants. In addition, the NIDA has targeted a portion of the funds available for competing research project grants to support high priority projects outside of the payline, including awards to new investigators, and early investigators. The Institute also seeks to maintain a balance between solicitations issued to the extramural community in areas that need stimulation and funding made available to support investigator-initiated projects. Intramural research and Research management and Support receive modest increases to help offset the cost of pay and other increases.
FY 2010 Justification by Activity Detail
Program Descriptions and Accomplishments
Basic and Clinical Neuroscience: Basic and Clinical neuroscience represent two programs in NIDA that work together to enlarge understanding of the neurobiological, genetic, and behavioral factors underlying drug abuse and addiction. Specifically, they examine the factors affecting increased risk and/or resilience to drug abuse, addiction, and drug-related disorders; the mechanisms of addiction; and the effects of drugs on the brain and behavior. To see these effects in real time, NIDA researchers are increasingly integrating brain imagining tools like functional magnetic resonance imaging (fMRI) into their studies. This may facilitate the development of novel treatments for addiction using "neurofeedback" (i.e., training patients to influence brain activation at specific sites) and will allow the examination of less-studied brain circuits, such as those involved with interoception (internal monitoring of bodily functions and sensations) linked with emotion and motivation. A greater understanding of interoceptive processing may lead to new targets for treatment research to reduce a patients' risk of relapse to substance abuse. Another emerging research area is epigeneticsŠŠthe study of long-term changes in gene function that result from environmental impacts, such as drug exposure, maternal behavior, and stress. This is the focus of a key NIH Roadmap initiative that NIDA co-leads with NIDCD and NIEHS. Collectively, this research provides the fundamental information to develop and communicate prevention and treatment interventions for drug abuse and addiction.
Budget Policy: The FY 2010 estimate for this program area is $491.018 million, an increase of $5.632 million and 1.2 percent above the FY 2009 estimate. By applying funds from grants that ended in FY 2008, we will pursue opportunities in line with our top priorities, one of which is exploring gene x drug interactions to better identify addiction vulnerability.
Portrait of a Program: Genetics and Addiction
FY 2009 Level: $107.165 million
FY 2010 Level: $105.895 million
Change: $1.270 million
Research has shown that drug abuse and addiction are complex diseases, resulting from the interplay of genes, environment, and developmental stage. Genetics accounts for approximately half of an individual's vulnerability to addiction, including the way genes interact with the environment and with developmental stage. Scientific advances have yielded tools of exceptional power to identify and use these variables to better tailor prevention and treatment strategies.
An important goal in understanding the role of genetics in addiction is to see how genes affect brain function and, in turn, influence behavior. To this end, NIDA supports genome wide association studies to establish reliable connections between particular genotypes and addiction risk. The results of a recent study involving nearly 11,000 Icelandic smokers found that variations in a gene cluster (harboring the α3, α5, and β4 nicotinic receptor subunits) had a strong effect on nicotine dependence and the risk of lung cancer and peripheral arterial disease. These findings are remarkable because (1) they converge with other, independently gathered data associating the same gene cluster with nicotine dependence or lung cancer susceptibility and (2) they reveal promising new targets for treating nicotine addiction and lung cancer.
Complementing these efforts, NIDA's pharmacogenomics research - the study of how genes regulate an individual's reaction to drugs and medications - can help treatment providers tailor medications for individual patients. NIDA is also working with other institutes and disciplines in building up the informatics and technological infrastructure to optimize the value of all the genetic data being generated.
FY 09 funding for genetics projects is spread across different NIDA funding mechanisms and therefore encompasses different start dates and average lengths.
Epidemiology, Services and Prevention Research: This major program area seeks to promote integrated approaches to understand and address the interactions between individuals and environments that contribute to the continuum of drug abuseŠrelated problems. The vision is to support research and major data collection systems and surveillance networks to help identify substance abuse trends locally, nationally, and internationally; to guide development of responsive interventions for a variety of populations; and to encourage optimal service delivery in real-world settings.
By mining our National Monitoring the Future Survey of youth, a robust correlation was discovered between regular exercise in 12th graders and lower prevalence rates of daily cigarette smoking or marijuana use in the past month. Spurred in part by this compelling finding, NIDA-sponsored a science meeting in June 2008, entitled "Can Physical Activity and Exercise Prevent Substance Use: Promoting a Full Range of Science to Inform Prevention." The meeting allowed scientists to share relevant research findings addressing the relationship between physical activity/exercise and behavioral health. NIDA has since issued a call for studies on physical activity and drug abuse prevention, which will include basic, clinical, and services research.
Budget Policy: The FY 2010 estimate for this program area is $246.140 million, an increase of $2.823 million and 1.2 percent above the FY 2009 estimate. A major focus for this NIDA program area is to improve drug abuse prevention and treatment services among populations including targeted research on drug abuse and stress among military service personnel and their families. NIDA will also reach out to major academic health centers that are part of the Clinical and Translational Science Awards (CTSA) consortium to ensure that drug abuse research is an integral component.
Pharmacotherapies and Medical Consequences: This program area is responsible for medications development aimed at helping people recover from drug abuse and addiction and sustain abstinence. Capitalizing on research showing the involvement of different brain systems in drug abuse and addictionŠŠbeyond the dopamine systemŠŠNIDA's medications development program is pursuing a variety of newly defined targets and treatment approaches. This program area also seeks solutions addressing the medical consequences of drug abuse and addiction, including infectious diseases such as HIV. NIDA is exploring several areas with exciting implications for the future. These include individualized treatments based on a person's genetic makeup, new mechanisms for restoring an addicted person's capacity to appreciate natural rewards in lieu of drugs, and pharmacotherapies that use an immunization strategy to help prevent relapse to drugs, with cocaine and nicotine vaccines now undergoing safety and efficacy testing, respectively, in humans.
Budget Policy: The 2010 estimate for this program area is $118.546 million, an increase of $1.360 million and 1.2 percent above the FY 2009 estimate. Program plans for FY 2010 give highest priority to facilitating medications development for patients addicted to multiple substances of abuse, both licit and illicit. NIDA is also building on the promise of immunotherapies to develop vaccines for drugs of abuse.
Program Portrait: NIDA's International HIV/AIDS-Drug Abuse Efforts
FY 2009 Level: $312.901 million
FY 2010 Level: $317.829 million
Change: $4.928 million
The proportion of new HIV infections attributable to injection drug use has shown a continuing 3-decade decline in the United States, thanks in part to improved treatments for injection drug users (e.g., methadone and buprenorphine for heroin addiction). However, for many other countries, HIV/AIDS is still a growing and deadly epidemic, driven in large part by intravenous heroin use. NIDA research has shown that medically supervised detoxification, drug counseling, and treatment with opioid medications can sustain abstinence and reduce injection drug risk, fostering the adoption of effective treatments by countries once unconvinced.
Malaysia, for example, is coping with the second highest HIV prevalence rate among adults and the highest proportion of HIV cases from injection drug use. Historically, Malaysia did not permit use of opioid agonists or partial agonists - addiction medications that bind to the same brain receptors as heroin, but are long-acting and can suppress withdrawal symptoms without intoxication (e.g., methadone and buprenorphine). In 2003, research findings demonstrating the efficacy of buprenorphine in sustaining heroin abstinence, improving treatment retention, and decreasing injection drug use led to the approval of both methadone and buprenorphine for treating opioid addiction in Malaysia. Rapidly embraced by the country's medical community, these medications have resulted in tens of thousands of opiate-dependent patients receiving medical treatment, helping to stem the spread of HIV infection among this population.
Similar success stories are unfolding in Russia, China, and the Ukraine, where research-demonstrated effectiveness stands to ultimately transform national policy and reduce HIV spread. NIDA has also issued a call for studies to develop a heroin vaccine, which would use antibodies to block the drug's entry into the brain and thereby prevent relapse. This approach may be more acceptable to countries that reject the use of agonist medications.
Clinical Trials Network: NIDA's National Drug Abuse Treatment Clinical Trials Network (CTN), which now comprises 16 research nodes and more than 240 individual community treatment programs, serves 34 States, plus the District of Columbia and Puerto Rico. The CTN tests the effectiveness of new and improved interventions in real-life community settings with diverse populations. It also serves as a research and training platform to help NIDA respond to emerging public health areas. Currently, the CTN provides a research platform for more than 30 research grants and a training platform for 60+ research fellows and junior faculty. Upcoming activities include plans to evaluate the potential value of exercise as an add-on to inpatient treatment for substance abusers, and a clinical trial to assess the relative effectiveness of various HIV testing strategies in reducing risky sexual and drug-related behaviors. Finally, in the wake of encouraging results in 2008 on the application of "Positive Choice", an interactive, patient-tailored computer program to improve clinic-based assessment and counseling for risky behaviors, NIDA is also planning to support the development of web-based training on addiction medicine for pain management providers.
Budget Policy: The FY 2010 estimate for this program area is $41.972 million, an increase of $482 thousand and 1.2 percent above the FY 2009 estimate. Program plans, along with expected accomplishments, are a continuation of initiatives begun in FY 2008 to (1) assess the effectiveness of a 12-step facilitation intervention for stimulant abusing patients in initiating and sustaining their involvement with support groups like Cocaine or Alcoholics Anonymous, (2) determine whether adding individual drug counseling to buprenorphine/naloxone (BUP/NX) treatment, along with Standard Medical Management (SMM), improves outcomes for patients addicted to pain medications, and (3) compare the effect of BUP/NX versus methadone on liver enzymes in patients entering opioid treatment programs, a phase 4 study requested by the FDA to provide additional information on risks, benefits, and optimal use of these medications.
Intramural Research Program (IRP): This Intramural program performs cutting edge research within a coordinated multidisciplinary framework. The IRP attempts to elucidate the nature of the addictive process; to determine the potential use of new therapies for substance abuse, both pharmacological and psychosocial; and to decipher the long-term consequences of drugs of abuse on brain development, maturation, function, and structure, and on other organ systems. Recent IRP activities include the conduct of basic research to understand the role of mitochondriaŠŠthe "powerhouse" of a cell that breaks down glucose to release energy - in degenerative neurological diseases (e.g., Parkinson's Disease). IRP activities also use a variety of animal models of addiction to better understand the effects of drugs on brain and behavior. In addition, the IRP supports an HIV/AIDS Pathophysiology and Medications Discovery Program, which focuses on (1) how HIV or its products cross the blood-brain barrier, (2) how toxic compounds generated by HIV invade brain cells, and (3) the development of compounds to block the toxic effects of HIV on immune system cells.
Budget Policy: The FY 2010 estimate for this program area is $87.566 million, an increase of $1.294 million and 1.5 percent above the FY 2009 estimate. NIDA plans to take advantage of new and emerging techniques, including new tools to measure the effect of psychosocial stress on individuals with substance-use disorders by collecting behavioral and physiological data in participants' real time environments. This activity represents the first systematic, prospective effort to link indices of community-level risk to intensive field measurements of individual attempts at behavior change.
Research Management and Support (RMS): RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. Additionally, the functions of RMS encompass strategic planning, coordination, and evaluation of NIDA's programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public. NIDA currently oversees more than 1,800 research grants and more than 190 research and development contracts.
In addition to the infrastructure required to support research and training, NIDA also strives to educate the public about drug abuse and addiction and to raise awareness of the science behind it. In October 2008, NIDA held its second Drug Facts Chat Day, following an overwhelming response the year before, to again give students and teachers across the country the chance to interact with NIDA staff via the Internet on questions about drugs' effects on the brain and body, and a variety of other issues related to addiction and treatment. NIDA also strives to encourage young people interested in careers in science. This year NIDA co-sponsored an Addiction Science award given at the Intel International Science and Engineering Fair to the top three projects advancing addiction science. The winners offered impressive and innovative approaches to exploring some of the neurological and environmental underpinnings of addiction. This premier event was followed by an in-person visit to NIDA and NIH by the top winners, who presented their findings to staff and to Dr. Elias Zerhouni, who was then director of NIH.
Budget Policy: The FY 2010 estimate for this program area is $60.142 million, an increase of $1.034 million and 1.7 percent above the FY 2009 estimate. NIDA will continue to support scientific meetings to stimulate interest and develop research agendas in areas significant to drug abuse and addiction. NIDA will also continue to support educational outreach aimed at diverse audiences, including the general public, HIV high-risk populations, physicians, judges, and educators to help raise awareness of substance abuse issues and disseminate promising prevention and treatment strategies.
NIH Common Fund: NIDA is the co-lead, with NIEHS and NIDCD on the Epigenomics initiative; and with OBSSR on the initiative: Facilitating Interdisciplinary Research Via Methodological and Technological Innovation in the Behavioral and Social Sciences. NIDA also participates in the support of Institutional Training Grants focused on Interdisciplinary training through the NIH Blueprint and the NIH Common Fund.
| FY 2009 Estimate | FY 2010 PB | Increase or Decrease | |
|---|---|---|---|
| Total compensable work years: | |||
| Full-time employment | 384 | 392 | 8 |
| Full-time equivalent of overtime and holiday hours | 0 | 0 | 0 |
| Average ES salary | $158,223 | $161,387 | $3,164 |
| Average GM/GS grade | 12.8 | 12.8 | 0.0 |
| Average GM/GS salary | $104,086 | $106,167 | $2,081 |
| Average salary, grade established by act of July 1, 1944 (42 U.S.C. 207) | $92,111 | $93,953 | $1,842 |
| Average salary of ungraded positions | 115,063 | 117,365 | 2,302 |
| OBJECT CLASSES | |||
| Personnel Compensation: | |||
| 11.1 Full-time permanent | $27,594,000 | $28,870,000 | $1,276,000 |
| 11.3 Other than full-time permanent | 10,206,000 | 10,776,000 | 570,000 |
| 11.5 Other personnel compensation | 1,473,000 | 1,546,000 | 73,000 |
| 11.7 Military personnel | 1,331,000 | 1,398,000 | 67,000 |
| 11.8 Special personnel services payments | 2,054,000 | 2,176,000 | 122,000 |
| Total, Personnel Compensation | 42,658,000 | 44,766,00 | 2,108,00 |
| 12.0 Personnel benefits | 9,841,000 | 10,326,000 | 485,000 |
| 12.2 Military personnel benefits | 868,000 | 912,000 | 44,000 |
| 13.0 Benefits for former personnel | 0 | 0 | 0 |
| Subtotal, Pay Costs | 53,367,000 | 56,004,000 | 2,637,000 |
| 21.0 Travel and transportation of persons | 1,285,000 | 1,249,000 | (36,000) |
| 22.0 Transportation of things | 111,000 | 109,000 | (2,000) |
| 23.1 Rental payments to GSA | 0 | 0 | 0 |
| 23.2 Rental payments to others | 32,000 | 31,000 | (1,000) |
| 23.3 Communications, utilities and miscellaneous charges | 831,000 | 809,000 | (22,000) |
| 24.0 Printing and reproduction | 687,000 | 662,000 | (25,000) |
| 25.1 Consulting services | 7,831,000 | 7,740,000 | (91,000) |
| 25.2 Other services | 4,545,000 | 4,438,000 | (107,000) |
| 25.3 Purchase of goods and services from government accounts | 104,963,000 | 105,454,000 | 491,000 |
| 25.4 Operation and maintenance of facilities | 2,300,000 | 2,294,000 | (6,000) |
| 25.5 Research and development contracts | 56,783,000 | 57,760,000 | 977,000 |
| 25.6 Medical care | 198,000 | 198,000 | 0 |
| 25.7 Operation and maintenance of equipment | 852,000 | 847,000 | (5,000) |
| 25.8 Subsistence and support of persons | 0 | 0 | 0 |
| 25.0 Subtotal, Other Contractual Services | 177,472,000 | 178,731,000 | 1,259,000 |
| 26.0 Supplies and materials | 3,266,000 | 3,242,000 | (24,000) |
| 31.0 Equipment | 2,791,000 | 2,750,000 | (41,000) |
| 32.0 Land and structures | 0 | 0 | 0 |
| 33.0 Investments and loans | 0 | 0 | 0 |
| 41.0 Grants, subsidies and contributions | 792,910,000 | 801,790,000 | 8,880,000 |
| 42.0 Insurance claims and indemnities | 0 | 0 | 0 |
| 43.0 Interest and dividends | 7,000 | 7,000 | 0 |
| 44.0 Refunds | 0 | 0 | 0 |
| Subtotal, Non-Pay Costs | 979,392,000 | 989,380,000 | 9,988,000 |
| Total Budget Authority by Object | 1,032,759,000 | 1,045,384,000 | 12,625,000 |
| Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research | |||
| OBJECT CLASSES | FY 2009 Estimate | FY 2010 PB | Increase or Decrease |
|---|---|---|---|
| Personnel Compensation: | |||
| 11.1 Full-time permanent | $27,594,000 | $28,870,000 | $1,276,000 |
| 11.3 Other than full-time permanent | 10,206,000 | 10,776,000 | 570,000 |
| 11.5 Other personnel compensation | 1,473,000 | 1,546,000 | 73,000 |
| 11.7 Military personnel | 1,331,000 | 1,398,000 | 67,000 |
| 11.8 Special personnel services payments | 2,054,000 | 2,176,000 | 122,000 |
| Total, Personnel Compensation (11.9) | 42,658,000 | 44,766,00 | 2,108,00 |
| 12.1 Civialian Personnel benefits | 9,841,000 | 10,326,000 | 485,000 |
| 12.2 Military personnel benefits | 868,000 | 912,000 | 44,000 |
| 13.0 Benefits for former personnel | 0 | 0 | 0 |
| Subtotal, Pay Costs | 53,367,000 | 56,004,000 | 2,637,000 |
| 21.0 Travel and transportation of persons | 1,285,000 | 1,249,000 | (36,000) |
| 22.0 Transportation of things | 111,000 | 109,000 | (2,000) |
| 23.2 Rental payments to others | 32,000 | 31,000 | (1,000) |
| 23.3 Communications, utilities and miscellaneous charges | 831,000 | 809,000 | (22,000) |
| 24.0 Printing and reproduction | 687,000 | 662,000 | (25,000) |
| Other Contractual Services | 177,472,000 | 178,731,000 | 1,259,000 |
| 25.1 Advisory and assistance services | 7,831,000 | 7,740,000 | (91,000) |
| 25.2 Other services | 4,545,000 | 4,438,000 | (107,000) |
| 25.3 Purchases from government accounts | 87,581,000 | 87,980,000 | 399,000 |
| 25.4 Operation and maintenance of facilities | 2,300,000 | 2,294,000 | (6,000) |
| 25.7 Operation and maintenance of equipment | 852,000 | 847,000 | (5,000) |
| 25.8 Subsistence and support of persons | 0 | 0 | 0 |
| Subtotal Other Contractual Services | 103,109,000 | 103,299,000 | 190,000 |
| 26.0 Supplies and materials | 3,256,000 | 3,232,000 | (24,000) |
| Subtotal, Non-Pay Costs | 109,311,00 | 109,391,000 | 80,000 |
| Total, Administrative Costs | 162,678,000 | 165,395,00 | 2,717,000 |
| PHS Act/Other Citation | U.S. Code Citation | 2009 Amount Authorized | FY 2009/Estimate | 2010 Amount Authorized | FY 2010 PB | |
|---|---|---|---|---|---|---|
| Research and Investigation | Section 301 | 42§241 | Indefinite | $1,032,759,000 | Indefinite | $1,045,384,000 |
| National Institute on Drug Abuse | Section 402(a) | 42§281 | Indefinite | $1,032,759,000 | Indefinite | $1,045,384,000 |
| Total, Budget Authority | $1,032,759,000 | $1,045,384,000 |
| Fiscal year | Budget Estimate to Congress | House Allowance | Senate Allowance | Appropriation(1) |
|---|---|---|---|---|
| 2001 | 496,294,000(2) | 788,201,000 | 789,038,000 | 781,327,000 |
| Rescission | (331,000) | |||
| 2002 | 907,369,000 | 900,389,000 | 902,000,000 | 888,105,000 |
| Rescission | (372,000) | |||
| 2003 | 960,582,000 | 968,013,000 | 968,013,000 | 968,013,000 |
| Rescission | (6,292,000) | |||
| 2004 | 995,614,000 | 995,614,000 | 997,614,000 | 997,414,000 |
| Rescission | (6,641,000) | |||
| 2005 | 1,019,060,000 | 1,019,060,000 | 1,026,200,000 | 1,014,760,000 |
| Rescission | (8,341,000) | |||
| 2006 | 1,010,130,000 | 1,010,130,000 | 1,035,167,000 | 1,010,130,000 |
| Rescission | (10,101,000) | |||
| 2007 | 994,829,000 | 994,829,000 | 1,000,342,000 | 1,000,621,000 |
| 2008 | 1,000,365,000 | 1,015,559,000 | 1,022,594,000 | 1,018,493,000 |
| Rescission | (17,793,000) | |||
| Supplemental | 5,322,000 | |||
| 2009 | 1,001,672,000 | 1,035,997,000 | 1,029,539,000 | 1,032,759,000 |
| 2010 | 1,045,384,000 |
1 - Reflects enacted supplementals, rescissions, and reappropriations.
2 - Excludes funds for HIV/AIDS research activities consolidated in the NIH Office of AIDS Research.
| OFFICE/DIVISION | FY 2008 Actual | FY 2009 Estimate | FY 2010 PB |
|---|---|---|---|
| Office of the Director | 23 | 24 | 24 |
| Office of Extramural Affairs | 16 | 17 | 17 |
| Office of Management | 63 | 64 | 64 |
| Office of Science Policy & Communications | 30 | 31 | 31 |
| Division of Epidemiology, Services & Prevention Research | 30 | 31 | 33 |
| Division of Basic Neurosciences & Behavioral Research | 29 | 30 | 33 |
| Division of Pharmacotherapies & Medical Consequences of Drug Abuse | 33 | 34 | 35 |
| Center for the Clinical Trials Network | 13 | 14 | 15 |
| Division of Clinical Neuroscience & Behavioral Research | 16 | 17 | 18 |
| Intramural Research Program | 123 | 122 | 122 |
| Total | 376 | 384 | 392 |
| Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research FTEs supported by funds from Cooperative Research and Development Agreements | (0) | (0) | (0) |
| FISCAL YEAR | Average GM/GS Grade |
|---|---|
| 2006 | 12.5 |
| 2007 | 12.6 |
| 2008 | 12.8 |
| 2009 | 12.8 |
| 2010 | 12.8 |
| GRADE | FY 2008 Actual | FY 2009 Estimate | FY 2010 PB |
|---|---|---|---|
| Total, ES Postions | 1 | 1 | 1 |
| Total, ES Salary | 155,121 | 158,223 | 161,387 |
| GM/GS-15 | 63 | 63 | 63 |
| GM/GS-14 | 93 | 93 | 93 |
| GM/GS-13 | 55 | 55 | 55 |
| GS-12 | 47 | 47 | 47 |
| GS-11 | 11 | 11 | 11 |
| GS-10 | 2 | 2 | 2 |
| GS-9 | 12 | 12 | 12 |
| GS-8 | 9 | 9 | 9 |
| GS-7 | 9 | 9 | 9 |
| GS-6 | 2 | 2 | 2 |
| GS-5 | 3 | 3 | 3 |
| GS-4 | 1 | 1 | 1 |
| GS-3 | |||
| GS-2 | |||
| GS-1 | |||
| SubTotal | 307 | 307 | 307 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): | |||
| Assistant Surgeon General | |||
| Director Grade | 7 | 7 | 7 |
| Senior Grade | 4 | 4 | 4 |
| Full Grade | |||
| Senior Assistant Grade | 1 | 1 | 1 |
| Assistant Grade | |||
| SubTotal | 12 | 12 | 12 |
| Ungraded | 78 | 78 | 78 |
| Total permanent positions | 317 | 317 | 317 |
| Total positions, end of year | 398 | 384 | 392 |
| Total full-time equivalent (FTE) employment, end of year | 376 | 384 | 392 |
| Average ES salary | 155,121 | 158,223 | 161,387 |
| Average GM/GS grade | 12.8 | 12.8 | 12.8 |
| Average GM/GS salary | 102,046 | 104,086 | 106,167 |
| Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research. | |||
New Positions Requested FY 2010
- Health Science Administrator, Grade GS-13/14, Number - 8, Annual Salary $100,939. Total Positions Requested - 8
