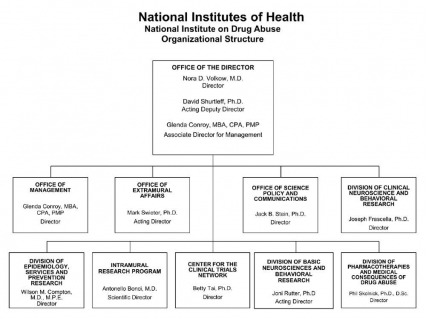
Organizational Chart
- Text description of organizational chart
-
Office of the Director
Nora D. Volkow, M.D., Director
David Shurtleff, Ph.D., Acting Deputy Director
Glenda Conroy, MBA, CPA, PMP, Associate Director for ManagementOffice of Management
Glenda Conroy, MBA, CPA, PMP, DirectorOffice of Extramural Affairs
Mark Swieter, Ph.D., Acting DirectorOffice of Science Policy and Communications
Jack B. Stein, Ph.D., DirectorDivision of Clinical Neuroscience and Behavioral Research
Joseph Frascella, Ph.D., DirectorDivision of Epidemiology, Services and Prevention Research
Wilson M. Compton, M.D., M.P.E., DirectorIntramural Research Program
Antonello Bonci, M.D., Scientific DirectorCenter for the Clinical Trials Network
Betty Tai, Ph.D., DirectorDivision of Basic Neurosciences and Behavioral Research
Joni Rutter, Ph.D., Acting DirectorDivision of Pharmacotherapies and Medical Consequences of Drug Abuse
Phil Skolnick, Ph.D., D.Sc., Director
Appropriations Language
For carrying out section 301 and title IV of the PHS Act with respect to drug abuse, $1,071,612,000.
Tables
- Amounts Available for Obligation Table
-
Amounts Available for Obligation 1
(Dollars in Thousands)Source of Funding FY 2012 Actual FY 2013 CR FY 2014 PB Appropriation 1,055,362 1,059,814 1,071,612 Rescission (1,995) 0 0 Subtotal, adjusted appropriation 1,053,367 1,059,814 1,071,612 Secretary's Transfer for Alzheimer's disease (AD) (694) 0 0 Secretary's Transfer for AIDS authorized by PL 112-74, Section 206 (300) 0 0 Comparative Transfers to NLM for NCBI and Public Access (963) (1,247) 0 Subtotal, adjusted budget authority 1,051,410 1,058,567 1,071,612 Unobligated balance, start of year 0 0 0 Unobligated balance, end of year 0 0 0 Subtotal, adjusted budget authority 1,051,410 1,058,567 1,071,612 Unobligated balance lapsing (5) 0 0 Total obligations 1,051,405 1,058,567 1,071,612 1 Excludes the following amounts for reimbursable activities carried out by this account:
FY 2012 - $37,125 FY 2013 - $80,444 FY 2014 - $87,443
- Budget Mechanism Table
-
- Summary of Changes Table
-
Summary of Changes
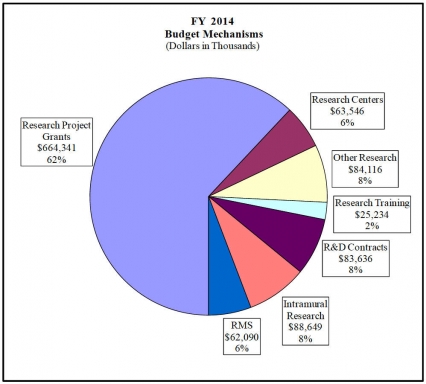
(Dollars in Thousands)FY 2012 Actual $1,051,410 FY 2014 President's Budget $1,071,612 Net change $20,202 CHANGES 2014 President's Budget: FTEs 2014 President's Budget: Budget Authority Change from FY 2012: FTEs Change from FY 2012: Budget Authority A. Built-in: 1. Intramural Research: a. Annualization of March 2013 pay increase & benefits $24,346 $66 b. January FY 2014 pay increase & benefits 24,346 178 c. One more day of pay 24,346 92 d. Differences attributable to change in FTE 24,346 0 e. Payment for centrally furnished services 11,229 203 f. Increased cost of laboratory supplies, materials, other expenses, and non-recurring costs 53,074 70 Subtotal $609 2. Research Management and Support: a. Annualization of March 2013 pay increase & benefits $38,637 $105 b. January FY 2014 pay increase & benefits 38,637 286 c. One more day of pay 38,637 146 d. Differences attributable to change in FTE 38,637 0 e. Payment for centrally furnished services 4,316 82 f. Increased cost of laboratory supplies, materials, other expenses, and non-recurring costs 19,137 3 Subtotal $622 Subtotal, Built-in $1,231 B. Program: 1. Research Project Grants: a. Noncompeting 1,060 $481,369 19 $14,471 b. Competing 423 159,866 24 -2,071 c. SBIR/STTR 63 23,106 7 2,865 Total 1,546 $664,341 50 $15,265 2. Research Centers 35 $63,546 -3 $0 3. Other Research 297 84,116 0 0 4. Research Training 548 25,234 -9 0 5. Research and development contracts 169 83,636 0 4,140 Subtotal, Extramural $920,873 $19,405 FTEs FTEs 6. Intramural Research 128 $88,649 4 $187 7. Research Management and Support 283 62,090 25 -622 8. Construction 0 0 9. Buildings and Facilities 0 0 Subtotal, program 411 $1,071,612 29 $18,970 Total changes $20,202
-
Authorizing Legistlation PHS Act/Other Citation U.S. Code Citation 2013 Amount Authorized FY 2013 CR 2014 Amount Authorized FY 2014 PB Research and Investigation Section 301 42§241 Indefinite $1,058,567,000 Indefinite $1,071,612,000 National Institute on Drug Abuse Section 401(a) 42§281 Indefinite Indefinite Total, Budget Authority $1,058,567,000 $1,071,612,000
- Appropriations History Table
-
Appropriations History Fiscal Year Budget Estimate to Congress House Allowance Senate Allowance Appropriation 2005 $1,019,060,000 $1,019,060,000 $1,026,200,000 $1,014,760,000 Rescission ($8,341,000) 2006 $1,010,130,000 $1,010,130,000 $1,035,167,000 $1,010,130,000 Rescission ($10,101,000) 2007 $994,829,000 $994,829,000 $1,000,342,000 $1,000,621,000 Rescission - 2008 $1,000,365,000 $1,015,559,000 $1,022,594,000 $1,018,493,000 Rescission ($17,793,000) Supplemental $5,322,000 2009 $1,001,672,000 $1,035,997,000 $1,029,539,000 $1,032,759,000 Rescission - 2010 $1,045,384,000 $1,069,583,000 $1,050,091,000 $1,059,848,000 Rescission - 2011 $1,094,078,000 - $1,092,369,000 $1,059,848,000 Rescission ($9,306,097) 2012 $1,080,018,000 $1,080,018,000 $1,038,714,000 $1,055,362,000 Rescission ($1,994,634) 2013 $1,054,001,000 - 1,057,196,000 - Rescission - 2014 $1,071,612,000 - - -
-
Budget Authority by Object Table
(Dollars in Thousands)FY 2012 Actual FY 2014 PB Increase or Decrease Full-time employment 382 411 29 Full-time equivalent of overtime and holiday hours 0 0 0 Average ES salary (in whole dollars) $177,884 $180,112 $2,228 Average GM/GS grade 12.8 12.7 (0.1) Average GM/GS salary (in whole dollars) $110,974 $108,133 ($2,841) Average salary, grade established by act of July 1, 1944 (42 U.S.C. 207) (in whole dollars) $110,901 $112,010 $1,109 Average salary of ungraded positions (in whole dollars) $140,659 $142,066 $1,407 OBJECT CLASSES FY 2012 Actual FY 2014 PB Increase or Decrease Personnel Compensation: 11.1 Full-time permanent $30,229 $32,072 $1,843 11.3 Other than full-time permanent 10,921 11,432 511 11.5 Other personnel compensation 813 849 36 11.7 Military personnel 1,173 1,214 41 11.8 Special personnel services payments 4,222 4,275 53 Total, Personnel Compensation $47,359 $49,842 $2,483 12.0 Personnel benefits $11,771 $12,292 $521 12.2 Military personnel benefits 691 715 24 13.0 Benefits for former personnel 0 0 0 Subtotal, Pay Costs $59,821 $62,849 $3,028 21.0 Travel and transportation of persons $938 $1,210 $272 22.0 Transportation of things 75 75 0 23.1 Rental payments to GSA 5 5 0 23.2 Rental payments to others 26 26 0 23.3 Communications, utilities and miscellaneous charges 1,222 1,222 0 24.0 Printing and reproduction 13 13 0 25.1 Consulting services 2,909 2,067 (842) 25.2 Other services 3,704 3,241 (463) 25.3 Purchase of goods and services from government accounts 107,750 110,461 2,711 25.4 Operation and maintenance of facilities 2,100 1,931 (169) 25.5 Research and development contracts 43,263 44,638 1,375 25.6 Medical care 1,931 1,728 (203) 25.7 Operation and maintenance of equipment 1,496 1,496 0 25.8 Subsistence and support of persons 0 0 0 25.0 Subtotal, Other Contractual Services $163,153 $165,562 $2,409 26.0 Supplies and materials $3,522 $4,374 $852 31.0 Equipment 5,411 4,821 (590) 32.0 Land and structures 0 0 0 33.0 Investments and loans 0 0 0 41.0 Grants, subsidies and contributions 817,225 831,455 14,230 42.0 Insurance claims and indemnities 0 0 0 43.0 Interest and dividends 0 0 (0) 44.0 Refunds 0 0 0 Subtotal, Non-Pay Costs $991,589 $1,008,763 $17,174 Total Budget Authority by Object $1,051,410 $1,071,612 $20,202 Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
- Salaries and Expenses Table
-
Salaries and Expenses
(Dollars in Thousands)OBJECT CLASSES FY 2012 Actual FY 2014 PB Increase or Decrease Personnel Compensation: Full-time permanent (11.1) $30,229 $32,072 $1,843 Other than full-time permanent (11.3) 10,921 11,432 511 Other personnel compensation (11.5) 813 849 36 Military personnel (11.7) 1,173 1,214 41 Special personnel services payments (11.8) 4,222 4,275 53 Total Personnel Compensation (11.9) $47,358 $49,842 $2,484 Civilian personnel benefits (12.1) $11,771 $12,292 $521 Military personnel benefits (12.2) 691 715 24 Benefits to former personnel (13.0) 0 0 0 Subtotal, Pay Costs $59,820 $62,849 $3,029 Travel (21.0) $938 $1,210 $272 Transportation of things (22.0) 75 75 0 Rental payments to others (23.2) 26 26 0 Communications, utilities and miscellaneous charges (23.3) 1,222 1,222 0 Printing and reproduction (24.0) 13 13 0 Other Contractual Services: Advisory and assistance services (25.1) 2,909 2,067 (842) Other services (25.2) 3,704 3,241 (463) Purchases from government accounts (25.3) 70,922 67,846 (3,076) Operation and maintenance of facilities (25.4) 2,100 1,931 (169) Operation and maintenance of equipment (25.7) 1,496 1,496 0 Subsistence and support of persons (25.8) 0 0 0 Subtotal Other Contractual Services $81,131 $76,581 ($4,550) Supplies and materials (26.0) $3,001 $3,852 $851 Subtotal, Non-Pay Costs $86,406 $82,979 ($3,427) Total, Administrative Costs $146,226 $145,828 ($398)
- Detail of Full-Time Equivalent Employment (FTEs) Table
-
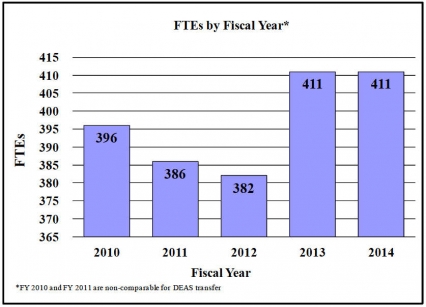
Details of Full-Time Equivalent Employment (FTEs) FY 2012 Actual FY 2013 CR FY 2014 PB OFFICE/DIVISION Civilian Military Total Civilian Military Total Civilian Military Total Office of the Director Direct: 23 0 23 24 0 24 23 0 23 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 23 0 23 24 0 24 23 0 23 Office of Extramural Affairs Direct: 17 0 17 22 0 22 22 0 22 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 17 0 17 22 0 22 22 0 22 Office of Management Direct: 50 0 50 55 0 55 55 0 55 Reimbursable: 23 0 23 26 0 26 28 0 28 Total: 73 0 73 81 0 81 83 0 83 Office of Science Policy and Communication Direct: 28 0 28 28 0 28 28 0 28 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 28 0 28 28 0 28 28 0 28 Division of Epidemiology, Services & Prevention Research Direct: 26 3 29 28 2 30 28 2 30 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 26 3 29 28 2 30 28 2 30 Division of Basic Neuroscience & Behavioral Research Direct: 28 0 28 32 0 32 32 0 32 Reimbursable: 1 0 1 1 0 1 1 0 1 Total: 29 0 29 33 0 33 33 0 33 Division of Pharmacotherapies and Medical Consequences of Drug Abuse Direct: 29 0 29 32 0 32 32 0 32 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 29 0 29 32 0 32 32 0 32 Center for the Clinical Trials Network Direct: 13 2 15 16 1 17 16 1 17 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 13 2 15 16 1 17 16 1 17 Division of Clinical Neuroscience & Behavioral Research Direct: 15 0 15 16 0 16 15 0 15 Reimbursable: 0 0 0 0 0 0 0 0 0 Total: 15 0 15 16 0 16 15 0 15 Intramural Research Program Direct: 116 6 122 120 6 126 120 6 126 Reimbursable: 2 0 2 2 0 2 2 0 2 Total: 118 6 124 122 6 128 122 6 128 Total (Includes FTEs whose payroll obligations are supported by the NIH Common Fund) 371 11 382 402 9 411 402 9 411 FTEs supported by funds from Cooperative Research and Development Agreements 0 0 0 0 0 0 0 0 0 Fiscal Year Average GS Grade 2010 13.0 2011 13.0 2012 12.8 2013 12.7 2014 12.7
- Detail of Positions Table
-
Detail of Positions GRADE FY 2012 Actual FY 2013 CR FY 2014 PB Total, ES Positions 1 1 1 Total, ES Salary $177,884 $178,329 $180,112 GM/GS-15 66 66 66 GM/GS-14 87 87 87 GM/GS-13 58 58 58 GS-12 38 39 39 GS-11 13 13 13 GS-10 1 1 1 GS-9 16 16 16 GS-8 13 17 17 GS-7 4 17 17 GS-6 0 3 3 GS-5 2 3 3 GS-4 4 4 4 GS-3 1 1 1 GS-2 0 0 0 GS-1 0 0 0 Subtotal 303 325 325 Grades established by Act of July 1, 1944 (42 U.S.C. 207): Assistant Surgeon General 0 0 0 Director Grade 0 0 0 Senior Grade 8 7 7 Full Grade 0 0 0 Senior Assistant Grade 1 1 1 Assistant Grade 0 0 0 Subtotal 9 8 8 Ungraded 70 70 70 Total permanent positions 306 335 335 Total positions, end of year 383 412 412 Total full-time equivalent (FTE) at YE 382 411 411 Average ES salary $177,884 $178,329 $180,112 Average GM/GS grade 12.8 12.7 12.7 Average GM/GS salary $110,974 $107,062 $108,133 Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
Major Changes in the Fiscal Year 2014 President's Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanisms and activity detail and these highlights will not sum to the total change for the FY 2014 President's Budget for NIDA, which is $20.2 million more than the FY 2012 level, for a total of $1,071.6 million.
Research Project Grants (+$15.265 million; total $664.3 million): NIDA will support a total of 1,546 Research Project Grant (RPG) awards in FY 2014. Non-competing RPGs will increase by 19 awards and $16.245 million. Competing RPGs will increase by 24 awards and decrease in the amount of $2.071 million. Within the total, research priorities include those that position the Institute to advance the development of medications, using innovative genetics tools and technologies, and translating the results of evidence-based findings to improve drug abuse interventions and promote greater access to them worldwide. NIDA will also continue to make the support of new and early stage investigators a priority.
HIV/AIDS Research (+$12.994 million; total $332.3 million): NIDA plans to support targeted HIV/AIDS research in FY 2014. Efforts will center on particularly vulnerable populations such as ethnic minorities, homeless populations, and aging drug users. NIDA will also support HIV/AIDS research to advance proven interventions and their integration into primary medical systems.
Budget Graphs
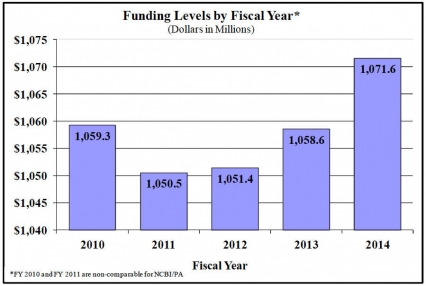
History of Budget Authority and FTEs:
Distribution by Mechanism:
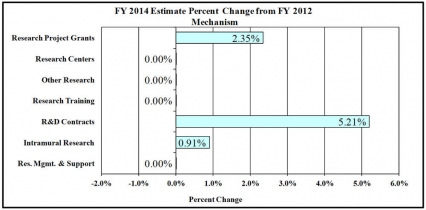
Change in Selected Mechanisms:
Justification of Budget Request
National Institute on Drug Abuse
Authorizing Legistlation: Section 301 and title IV of the Public Health Service Act, as amended.
| FY 2012 Actual | FY 2013 CR | FY 2014 President's Budget | FY 2014 +/- FY 2012 | |
|---|---|---|---|---|
| BA | 1,051,410,000 | 1,058,567,000 | 1,071,612,000 | +$20,202,000 |
| FTE | 382 | 411 | 411 | -29 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Director's Overview
Enlarging Our View of Addiction
The societal impact of substance abuse (alcohol, tobacco, illicit, and nonmedical use of prescription drugs) in this country is daunting, exceeding $600 billion a year in health care, crime-related, and productivity losses. To provide a comprehensive public health response, the National Institute on Drug Abuse (NIDA) will build on science advances from investments in genetics, pharmacotherapy, neuroscience, and behavioral and health services research that have led to innovative strategies for preventing and treating substance abuse and addiction.
NIDA will also pursue a "functional integration" or collaborative framework to enhance and expand activities related to substance use, abuse, and addiction research across the NIH, especially with the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Significant progress has already been made in the integration of NIDA's and NIAAA's intramural research programs and in the initiation of joint National Advisory Council meetings. This functional integration provides an important opportunity to pool resources and expertise to more effectively capitalize on synergies in addiction research, address scientific opportunities, and meet public health needs, while maintaining the unique research contributions of each IC.
The scope of both licit and illicit substance abuse is broad in this country. Prescription drug abuse, for example, remains a major concern across age groups and causes thousands of needless overdose deaths each year.1 Rates of marijuana use also hover at unacceptably high levels, even as new research reveals it can disrupt learning circuitry and lower IQ.2 Drugs compromise our young people's potential and the competitiveness of a nation that depends on them.
Basic Science for Tomorrow's Breakthroughs
Stunning discoveries in addiction research have enhanced understanding of the genetic, developmental, structural, and environmental factors that influence brain function in health and disease. Epigenetics—the study of how environmental factors can persistently alter gene expression (but not the DNA sequence) and thereby influence brain function and behavior— seeks to understand such phenomena. Animal studies have shown that factors like stress, drug exposure, and parenting behavior can influence addiction risk long-term through chemical modifications (i.e., epigenetic marks) in specific genes that may persist across generations: a recent study found that paternal cocaine exposure caused reduced body weight and changes in the brain systems governing mood regulation in the offspring. Epigenetics, combined with genetic information, will improve our ability to prevent, identify, diagnose, and treat addiction.
NIDA's multipronged strategy has helped usher in progress on several fronts. For one, it has led to innovation in medications development, such as anti-drug enzymes or antibodies to sequester the psychoactive ingredient of an abused substance in the bloodstream, slowing or preventing its entry into the brain (see Program Portrait on gene therapy). Also important is the search for predictive biomarkers to help clinicians select and monitor a therapeutic course of action and to help researchers evaluate and refine new therapeutic interventions. A recent advance showed, for example, that a person's response to treatment using a dopamine receptor drug can be predicted by individual differences in the levels of an enzyme (catecholamine o-methyl transferase) or of dopamine receptor type 3 expressed in white blood cells. Further, imaging studies of the brain at rest are revealing reproducible changes in brain structure and connectivity in addicted individuals—advances to help realize the promise of personalized medicine, allow for earlier identification and treatment of addicted patients, and reveal new targets for pharmacotherapies, desperately needed for addiction.
Public Health Impact: Translating Research to Real-World Practice
Translating findings from basic science into intervention strategies is not only key to exploring new approaches, but also to ensuring that evidence-based practices work in real-world settings, including a variety of health care venues and the criminal justice system. NIDA's National Drug Abuse Clinical Trials Network (CTN) and Criminal Justice-Drug Abuse Treatment Studies (CJDATS) enhance the rapid deployment of research-based interventions in multiple settings, undertaking studies that lead to more effective and cost-effective treatment approaches. To help integrate addiction services across the healthcare continuum, NIDA supports studies to identify barriers to and facilitators for integrating these services in diverse primary care settings.
Healthcare reform legislation portends needed change in this regard, presenting opportunities to study how innovations in service delivery, organization, and financing can be used to improve access to and use of effective prevention and treatment interventions (see Program Portrait on HIV). One area primed for change is screening, brief intervention, and referral to treatment (SBIRT) for substance use; NIDA will build on the success of screening for alcohol and tobacco use in primary care settings and continue to evaluate and expand SBIRT for other drugs of abuse and for special populations, including polydrug users and adolescents.
Another urgent public health issue pertains to substance abuse and associated comorbidities among military personnel, veterans, and their families. In collaboration with the Departments of Defense (DoD) and Veteran's Affairs (VA), NIAAA, and National Institute of Child Health and Human Development (NICHD), NIDA is launching a new initiative to develop and test interventions to prevent the onset and progression of drug abuse and comorbid conditions.
Other critical target populations for NIDA research include at-risk adolescents, such as those in the criminal justice system, with rates of substance abuse (or mental illness) that exceed 50 percent.3 In response, NIDA will support research to discover how juvenile justice systems can effectively implement a continuum of evidence-based substance abuse prevention and treatment services so as to reach every juvenile in the system and link them appropriately.
The Research Workforce: Encouraging Innovation
Attracting and retaining scientists can be challenging. NIDA will support the training and career development of a diverse pool of scientists to address all aspects of substance abuse and its health consequences. To meet the needs of modern science, NIDA will continue to create programs to attract researchers from physics, bioinformatics, and computational neuroscience— scientists who can create the needed language, tools, expertise, and infrastructure for researchers and clinicians to harmonize and analyze complex systems and large datasets. In this regard NIDA has taken the lead on several training initiatives supported through the NIH Neuroscience Blueprint—including Institutional grants in Computational Neuroscience and Neuroimaging. NIDA also offers special funding opportunities to facilitate the entry of much needed chemists into the drug abuse field through a program known as E-Chem, designed to help new-to-NIH researchers conduct exploratory research.
Overall Budget Policy: The FY 2014 President's Budget request for NIDA is $1.072 billion, an increase of $20 million or 1.9 percent above the FY 2012 Actual level. Research priorities include those that position the Institute to advance the development of medications, taking advantage of innovative genetics tools and technologies, and translating the results of evidencebased findings to improve drug abuse interventions and promote greater access to them worldwide. HIV prevention and treatment is another top NIDA research priority, including research on the interactions between HIV, substance abuse, and other comorbid psychiatric disorders, linking vulnerable populations to HIV prevention, testing, and treatment services, addressing HIV/AIDS-related health disparities, and integrating the treatment of substance abuse and HIV. Funds are included in R&D contracts to support trans-NIH initiatives, such as the Basic Behavioral and Social Sciences Opportunity Network (OppNet).
Program Descriptions and Accomplishments
Basic and Clinical Neuroscience: The Basic and Clinical Neuroscience programs work together to expand understanding of the neurobiological, genetic/epigenetic, and behavioral factors that underlie drug abuse and addiction. Specifically, they examine which variables influence risk of drug abuse, addiction, and drug-related disorders; how addiction works in the brain, including the effects of drugs on the expression or silencing of genes; and how resultant changes affect brain function and consequent behaviors. Research provides critical information to develop and test novel prevention and treatment interventions for drug abuse and addiction. New research will build on the growing recognition of the many essential roles played by nonneuronal cells (collectively called glia) in neurotransmission, inflammation, neuroprotection, and brain energy management. Recent findings suggest that glia can influence behavioral responses to drugs, including their rewarding or aversive effects. Also, HIV infection can undermine glial cells' vital role as supporters of neuronal physiology. NIDA is encouraging research to investigate the molecular and cellular correlates and consequences of substance abuse, HIV infection, and their interactions with glial cells in the central nervous system.
Budget Policy: The FY 2014 President's Budget estimate for this program is $465.3 million, an increase of $6.3 million, or 1.4 percent above the FY 2012 Actual level.
Epidemiology, Services and Prevention Research: This NIDA Division supports integrated approaches to understand and address the interactions between individuals and environments that contribute to drug abuse problems. It supports large surveys (e.g., the annual Monitoring the Future Survey, which tracks drug use and related attitudes among teens) and surveillance networks (e.g., the Community Epidemiology Work Group) to monitor drug-related issues and trends locally, nationally, and internationally; guide development of responsive interventions for a variety of populations; and encourage optimal service delivery in real-world settings (see Program Portrait). Key program areas include epidemiological studies on topics of special concern to better tailor prevention and treatment programs and adapt evidence-based interventions for target populations, such as youth, military, and the homeless. Runaways and other homeless young people are highly vulnerable and at greater risk for HIV infection and substance abuse. To address this critical public health gap, NIDA plans to support research to better understand the extent of HIV infection among homeless youth, the factors driving it, and how optimally to link these youth to HIV prevention, testing, care and treatment services.
In addition, this NIDA Division oversees partnering initiatives, including the first large-scale NIH-U.S. Food and Drug Administration (FDA) collaboration on tobacco regulatory research since Congress granted FDA the authority to regulate tobacco products. The Population Assessment of Tobacco and Health (PATH) Study, a national, longitudinal cohort study, will follow an estimated 59,000 adults and youth ages 12 to 18 to assess susceptibility to tobacco use, risk perceptions, use patterns, and resultant health impacts. Data collection is slated to begin in fall 2013, with plans for four or more annual data collection waves. Outcomes will inform current and future regulatory options for the FDA to protect public health, including setting tobacco product standards and communicating the risks of tobacco use to the general public.
Program Portrait: Potential of Healthcare Reform to Better Integrate HIV and Substance Abuse Treatment
FY 2012 Level: $10.1 million
FY 2014 Level: $11.6 million
Change: +$1.5 million
The stubbornly high incidence of new HIV infections in the United States (http://www.cdc.gov/hiv/statistics/index.html) belies the overwhelming evidence showing that sustained antiretroviral therapy (ART) provided to HIV+ individuals can improve the health of the infected individual and also prevent the spread of HIV to others. Moreover, in HIV+ substance users, widespread use of ART has been shown to reduce viral load and HIV incidence at the population level. Substance users contribute to HIV infection rates through injection drug use and high-risk sexual behaviors. Approximately one-third of the estimated 1.2 HIV-infected Americans currently use drugs or binge on alcohol. These individuals often do not receive ART treatment—partly because of concerns about possible non-compliance with the treatment regimens, or because they remain largely out of the treatment and care system. To reverse such trends will require a sustained and focused effort to seek, test, treat, and retain in care hard-to-reach substance users and to integrate substance abuse treatment with HIV treatment programs.
The Affordable Care Act of 2010 (ACA) and other healthcare reform legislation has the potential to lead needed change by providing medical insurance to a large percentage of currently uninsured persons with substance use disorders and by creating structural changes in the health care system that facilitate integration of substance abuse treatment with HIV programs. NIDA is exploring efficient and cost-effective models to test strategies to improve early HIV identification and immediate treatment for those who test positive—along with long-term HIV care— among drug users in both general medical and substance treatment settings.
Because of the high co-occurrence of substance abuse and infectious diseases such as HIV and Hepatitis C (HCV), infectious disease specialists also have a role to play in ensuring that their HIV+/HCV+ patients receive treatment for their substance use disorders. NIDA plans to support research to address this critical gap by understanding both the barriers to and opportunities for engaging infectious disease specialists in implementing screening, brief intervention, and referral to treatment in their practices. Identifying problems and starting and sustaining patients on needed treatment—for HIV and substance abuse—would have a significant impact on these intertwined epidemics in our country.
Budget Policy: The FY 2014 President's Budget estimate for this program is $264.4 million, an increase of $8.8 million, or 3.5 percent over the FY 2012 Actual level.
Pharmacotherapies and Medical Consequences: This program area is responsible for medications development aimed at helping people recover from drug abuse and addiction and sustain abstinence, and includes development of pain medications with less potential for abuse and addiction (see Program Portrait below). It capitalizes on research showing the involvement of different brain systems in drug abuse and addiction, beyond the pleasure pathway (the brain circuits engaged by activities that are pleasurable and that promote survival—e.g., eating, sex— and so tend to be repeated), to develop medications in response to a variety of newly defined targets. Joint efforts with the NIAAA will help NIDA expand its medication development efforts to exploit common addiction circuitry and address polysubstance abuse.
To get innovative compounds and delivery systems into the marketplace more quickly, NIDA works closely with industry and the FDA and supports public-private partnerships to advance effective addiction medications. The goal is to leverage the strengths of public (government agencies and institutes), non-profit (academia, NGOs, philanthropic institutions), and private sector entities to incentivize a steady flow of compounds that can more rapidly progress from "molecules to medicine." A related strategy to this end is a new funding paradigm (e.g., "GOMD" grants) aimed at "de-risking" medications in the early stages of discovery. This approach provides greater up-front support to closely monitored grantees for a shorter period of time, so that they can produce results quicker—whether for new compounds or repurposed medications— or change direction as needed.
This Division also supports innovative technological approaches to medication monitoring and delivery. For example, studies are being sought to develop and validate a reliable remote, realtime monitoring system to detect and measure cocaine ingestion in cocaine-dependent clinical trials paticipants. Such technology will be valuable for researchers testing and verifying treatments for cocaine addiction. This Division also includes programs to address the medical consequences of drug abuse and addiction, including Hepatitis C and HIV.
Program Portrait: Developing Pain Relievers with Less Abuse Liability
FY 2012 Level: $42.3 million
FY 2014 Level: $43.0 million
Change: +$0.7 million
Commonly prescribed for treating severe and/or chronic pain, opioid pain medications are beneficial when used appropriately. But since they act on the same receptors as heroin, they are also prone to abuse, with potentially dire consequences: CDC reports that since 1999, unintentional overdose deaths involving prescription opioid pain relievers have more than quadrupled in the United States.4 As the following examples illustrate, NIDA takes a multipronged approach to this problem that includes: (a) research to identify new analgesic compounds, targets, and drug combinations with reduced abuse, tolerance, and dependence risk; and (b) working with industry and government to devise alternative delivery systems and drug formulations that minimize diversion [use of a drug in a way that is not medically prescribed to obtain a "high" (e.g., inhaling or injecting crushed pills designed for oral treatment)] and prevent overdose deaths.
- Drugs that target the toll-like receptor 4 (TLR-4). TLR-4 is a type of immune detector located on nonneuronal cells called glia, which initiate an inflammatory response when activated. Recently these receptors were also shown to cause the loss of opioids' pain-relieving capacity with repeated use (i.e., tolerance) and to be involved in opioids' rewarding effects. Medications that block TLR-4 could both reverse opioid tolerance (and the need for higher doses) and negate rewarding effects. Clinical confirmation could result in a novel strategy to enhance the efficacy and reduce the addictiveness of pain medications.
- A pro-drug opioid medication. A pro-drug is one that is inactive in the bloodstream until it is cleaved by specific enzymes in the digestive system. It also does not readily cross the blood-brain barrier, thereby preventing abuse via non-oral routes (e.g., injection or inhalation). A pro-drug technology for an opioid medication has already sparked the interest of a small biotechnology company, which has begun pursuing its development, thereby positioning NIDA to partner with industry in supporting needed clinical studies.
- A drug formulation to reverse overdose from opioid drugs. Although naloxone (an opioid antagonist) is an effective medication for reversing overdose, it can only be administered by health care professionals. To broaden its availability and save lives, new formulations need to be approved for over-the-counter sale. NIDA will support research to advance the development of intranasal naloxone administration—specifically a clinical study to define doses that correspond to alternatively administered (e.g., injected) doses already approved by the FDA.
Given the scope of the prescription opioid abuse problem, developing analgesics, overdose treatments, and innovative methods of delivering them can and must quickly be translated into real-world use.
Program Portrait: Gene Therapy for Treating Stimulant Addiction
FY 2012 Level: $23.1 million
FY 2014 Level: $23.2 million
Change: +$0.1 million
Stimulant use disorders (e.g., cocaine, methamphetamine, prescription stimulants) remain a major public health concern. In 2011, over 1.8 million individuals currently (within the past month) abused either cocaine or methamphetamine.5 Unfortunately, no approved medications yet exist to treat addiction to stimulants. NIDA is funding a promising approach to treat stimulant and other substance use disorders that uses anti-drug enzymes or antibodies to neutralize the substance while it is still in the bloodstream, keeping it from entering the brain. The commercial feasibility of these approaches hinges on overcoming significant technical obstacles, one of which was addressed by two recent NIDA-supported preclinical studies that developed an attractive strategy based on the use of non-infective viruses modified to deliver (harmlessly) a gene that codes for the desired enzyme or antibody.
Preliminary results show that, once expressed in the body after a single injection of the virus carrier, these gene products can interfere with the pharmacological effects of the target drug for a long time. One study used an adenoassociated virus (AAV) to deliver a gene that encoded a high-affinity anti-cocaine monoclonal antibody to mice. The introduction of this gene stimulated the animals to produce high levels of cocaine-specific antibodies, which prevented cocaine from entering their brains and rendered them impervious to cocaine's behavioral effects for at least for 4 months after the injection. The second study used the same adenovirus system to deliver a gene that encodes for an enhanced version of a naturally occurring cocaine-metabolizing enzyme called butyrylcholinesterase. In rodents injected a single time with a virus carrying the gene for this enzyme, cocaine's effects in the body and central nervous system were completely blocked for at least 6 months.
These results suggest that using an adenovirus "shuttle" to deliver an efficient anti-cocaine antibody or cocainemetabolizing enzyme is feasible and could be used to treat cocaine addiction. NIDA proposes to extend the findings described, first to non-human primates and then via translation to the clinic. If successful, the AAV-based monoclonal antibody strategy could also be applied to other drugs of abuse (e.g., methamphetamine, heroin) where the feasibility of producing high-affinity monoclonal antibodies has already been demonstrated. If it proves successful, this delivery mechanism would obviate the need for patient adherence with frequent medication regimens or multiple vaccine injections.
Budget Policy: The FY 2014 President's Budget estimate for this program is $141.5 million, an increase of $4.0 million, or 2.9 percent over the FY 2012 Actual level.
Clinical Trials Network: NIDA's National Drug Abuse Treatment Clinical Trials Network (CTN) now comprises 13 research nodes and more than 240 individual community treatment programs in 38 States, plus the District of Columbia and Puerto Rico. The CTN works to develop treatment protocols for drug abuse and addiction and related conditions, such as comorbid mental health disorders and HIV, testing their real-world effectiveness with diverse patient populations and community treatment providers. It also serves as a research and training platform to help NIDA respond to emerging public health threats.
Since 1999, the CTN has increased the adoption of research-based treatments in community treatment practice. For example, CTN trials have shown that buprenorphine/naloxone is a safe and effective treatment for opioid addiction in young adults and in those abusing prescription opioid pain relievers. Buprenorphine/naloxone, which can be prescribed by qualified officebased physicians, is an alternative to methadone that can expand the reach of effective treatment for opioid addiction. Additionally, a CTN trial has shown that rapid HIV testing can be implemented in community-based drug abuse treatment centers, thus contributing to more comprehensive health care for patients with substance use disorders.
The CTN is currently at the final stage of completing (1) a multi-site study to evaluate Webdelivery of evidence-based psychosocial treatment for substance use disorders, (2) a trial of the safety and effectiveness of buprenorphine plus naltrexone for the treatment of cocaine addiction in patients also dependent on or abusing opioids, and (3) a trial evaluating the impact of adding smoking-cessation to standard treatment for stimulant addiction among patients who also smoke. Ongoing studies are evaluating the effect of screening, brief intervention, and referral to treatment (SBIRT) in emergency departments on substance use and substance-related outcomes, and a new study is comparing extended-release naltrexone (Vivitrol) to buprenorphine for patients addicted to heroin or other opioids, including prescription medications.
Budget Policy: The FY 2014 President's Budget estimate for this program is $49.6 million, an increase of $0.3 million, or 0.6 percent above the FY 2012 Actual level.
Intramural Research Program (IRP): The Intramural program performs cutting edge research within a coordinated multidisciplinary framework. The IRP attempts to (1) elucidate the nature of the addictive process; (2) determine the potential use of emerging new therapies for substance abuse, both pharmacological and psychosocial; and (3) establish the long-term consequences of drugs of abuse on systems and organs, with particular emphasis on the brain and its development, maturation, function, and structure. In addition, the IRP supports an HIV/AIDS Pathophysiology and Medications Discovery Program, focusing on, among other things, the development of compounds to block the destructive effects of HIV on immune system cells. IRP investigators are working on varied potential targets for addiction medications, many of which are being pursued through collaborative efforts with NIDA's extramural medications development program, an approach that should speed up the progress of selected targets along the NIDA medications development pipeline. In addition, NIDA and NIAAA together have made significant progress at integrating their intramural research programs in substance use, abuse, and addiction, including the appointment of a single Clinical Director for NIAAA and NIDA and the establishment of a joint genetics Intramural Research Program and a common optogenetics lab.
Budget Policy: The FY 2014 President's Budget estimate for this program is $88.6 million, an increase of $0.8 million, or 0.9 percent above the FY 2012 Actual level.
Research Management and Support (RMS): RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. Additionally, the functions of RMS encompass strategic planning, coordination, and evaluation of NIDA's programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public. NIDA currently oversees more than 1,800 research grants and more than 190 research and development contracts. In addition to the infrastructure required to support research and training, NIDA also strives to educate the public about drug abuse and addiction and to raise awareness of the science addressing it.
Indeed, physicians are a key target for NIDA's outreach efforts. In October 2012, the Office of National Drug Control Policy (ONDCP) and NIDA launched two online continuing medical education courses, using the "test-and-teach" model—one focused on safe prescribing for pain, the other on managing patients who abuse prescription opioids—in partnership with Medscape, a free web resource for physicians and other health professionals that features peer-reviewed original medical journal articles; medical news, major conference coverage, and drug information; and a customized version of the National Library of Medicine's MEDLINE database, among other things. After just 2 months, nearly 25,000 physicians and registered nurses have already completed these courses. In 2013, NIDA will publish a new NIDA Principles of Effective Treatment for Adolescents, intended to provide parents, referring clinicians, treatment practitioners, youth, and others with an evidence-based guide to the principles of effective substance abuse treatment for youth. This booklet complements Principles of Drug Addiction Treatment: A Research-Based Guide, highly regarded by the field. Like its parent, the new booklet offers FAQs, proven approaches, descriptions of multiple treatment modalities and settings, and a listing of other resources.
Budget Policy: The FY 2014 President's Budget estimate for this program is $62.1 million flat to the FY 2012 Actual level. The apparent increase in estimated FY 2014 FTE compared to the FY 2012 actual FTE usage level is due to the effect of transferring positions previously funded from a centralized support operation (Division of Extramural Activities Support) to individual ICs as of year-end 2012. As a result of the DEAS transfer, estimated salaries and benefits for FY 2014 are proportionately higher than those identified for FY 2012 and previous years.
NIH Collaborative Activities: NIDA actively participates in a variety of trans-NIH activities. NIDA has a lead role for the Common Fund–supported Epigenomics Program and additionally will be administering a request for application (RFA) to support a data management center for the new Common Fund Extracellular RNA Communication Program. NIDA participates in the NIH Neuroscience Blueprint—a collaboration between 15 NIH Institutes, Centers, and Offices that fund neuroscience research. It aims to accelerate neuroscientific discovery by pooling resources and expertise to address research challenges too large for any single institute or center. NIDA is the lead for the NIH Blueprint–supported Institutional Training Grants on Computational Neuroscience and Neuroimaging and the Neuroscience Information Framework (a dynamic inventory of Web-based neuroscience resources, data, and tools for scientists and students). NIDA also participates in the Basic Behavioral and Social Sciences Opportunity Network (OppNet) and in an OppNet-supported RFA titled the Effects of the Social Environment on Health, which will fund research to investigate structural, behavioral, sociocultural, environmental, cognitive, emotional, and/or biological mechanisms by which the social environment affects health outcomes. Additionally, NIDA collaborates and provides support for the HIV Prevention Trials Network (HPTN), funded by the Division of AIDS within the National Institute of Allergy and Infectious Diseases. HPTN focuses on the use of ART for HIV prevention and treatment, as well as treatment and prevention of sexually transmitted infections and substance abuse (particularly injection drug use) to reduce HIV transmission and acquisition.
NIDA has taken the lead, working with 20 NIH ICs and the Office of the Director, in supporting Centers of Excellence in Pain Education (CoEPEs). In May 2012, 12 CoEPEs were awarded to develop pain management curriculum resources for medical, dental, nursing, and pharmacy schools to advance the assessment, diagnosis, and safe treatment of pain, which will include a focus on minimizing risks of addiction to and diversion of opioid pain medications.
References
- Centers for Disease Control and Prevention, National Center for Health Statistics. Multiple Cause of Death 1999-2010 on CDC WONDER Online Database, released 2012.
- Meier et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. PNAS USA 109(40):E2657-64, 2012. PMID: 22927402.
- Wasserman et al. Psychiatric disorder, comorbidity, and suicidal behavior in juvenile justice youth. Criminal Justice and Behavior 37:1362, 2010.
- Centers for Disease Control and Prevention, National Center for Health Statistics. Multiple Cause of Death 1999- 2010 on CDC WONDER Online Database, released 2012.
- SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2011.