Drugs can alter the way people think, feel, and behave by disrupting neurotransmission, the process of communication between neurons (nerve cells) in the brain. Many scientific studies conducted over decades have established that drug dependence and addiction are features of an organic brain disorder caused by drugs’ cumulative impacts on neurotransmission. Scientists continue to build on this essential understanding with experiments to further elucidate the physiological factors that make a person prone to using drugs, as well as the full dimensions and progression of the disorder. The findings provide powerful leads for developing new medications and behavioral treatments.
This second article in our NIDA Notes Reference Series discusses the central importance of studying drugs’ effects on neurotransmission and describes some of the most common experimental methods used in this research. As with other articles in the series (see “Animal Experiments in Addiction Science”), we provide illustrative references from articles published in NIDA Notes.
What Is Neurotransmission?
A person reads. The words on the page enter the brain through the eyes and are converted into information that is relayed, from one neuron to the next, to regions that process visual input and attach meaning and memory. When inside neurons, the information takes the form of an electrical signal. To cross the tiny gap, or synapse, that separates one neuron from the next, the information takes the form of a chemical signal. The specialized molecules that carry the signals across the synapses are called neurotransmitters.
The ebb and flow of neurotransmitters—neurotransmission—is thus an essential feature of the brain’s response to experience and the environment. To grasp the basic idea of neurotransmission, think of a computer. A computer consists of basic units, semiconductors, which are organized into circuits; it processes information by relaying an electric current from unit to unit; the amount of current and its route through the circuitry determine the final output. The brain’s corresponding basic units are the neurons—86 billion of them. The brain relays information from neuron to neuron using electricity and neurotransmitters; the volume of these signals and their routes through the organ determine what we perceive, think, feel, and do.
Of course, the brain, a living organ, is much more complex and capable than any machine. Neurons respond with greater versatility to more types of input than any semiconductor; they also can change, grow, and reconfigure their own circuits.
Getting the Message Across
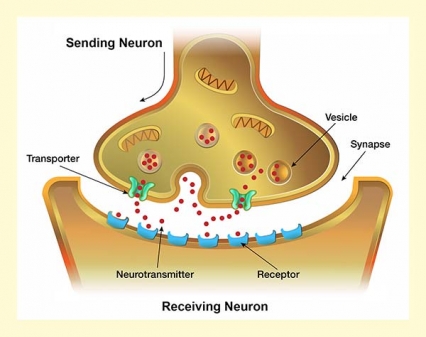
The task in neurotransmission is to convey a signal from a sending neuron to a receiving neuron across an open space known as a synapse. All neurons accomplish this in approximately the same way.
The sending cell manufactures neurotransmitter molecules and stores them in packets called vesicles. When stimulated sufficiently, the neuron generates an electric signal and causes some vesicles to migrate to the neuron membrane, merge with it, open up, and release their contents into the synapse. Some of the released molecules drift across the synapse and link up, lock-and-key fashion, with molecules called receptors on the surface of the receiving neuron. If the neurotransmitter is stimulatory (e.g., glutamate), its interaction with the receptor will raise the receiving neuron’s level of electrical activity and thereby increase the likelihood that it will, in turn, mobilize its vesicles and emit its own neurotransmitter. If the neurotransmitter is inhibitory (e.g., gamma-aminobutyric acid [GABA]), it will dampen the receiving neuron’s electrical activity and reduce its likelihood of releasing the neurotransmitter.
In this way, neurotransmitters relay information about the environment and our internal states from neuron to neuron through the brain’s circuits and, ultimately, shape how we respond. Neurotransmitters’ interactions with receptors can also set processes in motion that can alter the structure of receiving neurons, or raise (potentiate) or lower (depress) how strongly neurons respond when neurotransmitters link to their receptors in the future.
Once a neurotransmitter has interacted with its receptor on the receiving neuron, neuron to neuron communication is complete. The neurotransmitter molecules drop off the receptors. Loose again in the synapse, they meet one of three fates:
- Some attach to another receptor.
- Some encounter an enzyme, a chemical that breaks them apart.
- Some reenter the sending neuron via a special structure that spans the neuron membrane, called a transporter. Once back inside the neuron, they are available for re-release in future neurotransmission episodes.
Normally, when drugs are not present, the cycle of release, breakup, and neuron re-entry maintains the amount of neurotransmitter in the synapse, and hence neurotransmission, within certain limits. In most cases, when an addictive drug enters the brain, it causes neurotransmission to increase or decrease dramatically beyond these limits.
The Basic Research Questions
Neuroscientists seeking to understand why people use drugs and the consequences of drug use focus on two issues:
- Which neurotransmitter or neurotransmitters does the drug affect?
- How does the drug alter neurotransmission?
Which Neurotransmitter or Neurotransmitters Does the Drug Affect?
A person’s experiences when using a drug reflect the functional roles of the particular neurotransmitter(s) it disrupts. Each individual neuron manufactures one or more neurotransmitters: dopamine, glutamate, serotonin, acetylcholine, and/or any of dozens of others that scientists have identified to date. Each neurotransmitter is associated with particular effects depending on its distribution among the brain’s various functional areas (see Table 1). Dopamine, for example, is highly concentrated in regions that regulate motivation and feelings of reward, and is a strong motivator for drug use. A neurotransmitter’s impact also depends on whether it stimulates or dampens activity of its target neurons.
Some drugs primarily affect one neurotransmitter or class of neurotransmitters. For example, prescription opioids and heroin produce effects that are similar to (but more pronounced than) those produced by the neurotransmitters endorphin and enkephalin: increased analgesia, decreased alertness, and slowed respiration. Other drugs disrupt more than one type of neurotransmitter. Cocaine, for example, attaches to structures that regulate dopamine, leading to increases in dopamine activity and producing euphoria; it also produces changes in norepinephrine and glutamate systems that cause stimulant effects.
Because a neurotransmitter can stimulate or inhibit neurons that produce different neurotransmitters, a drug that disrupts one neurotransmitter can have secondary impacts on others. For example, nicotine stimulates cells directly by activating their receptors for acetylcholine, and indirectly by inducing higher levels of glutamate, a neurotransmitter that acts as an accelerator for neuron activity throughout the brain. A key effect that all drugs that cause dependence and addiction appear to have in common—a dramatic increase in dopamine signaling in a brain area called the nucleus accumbens (NAc), leading to euphoria and a desire to repeat the experience—is in many cases an indirect one.
- Table 1 - Neurotransmitters Implicated in Drug Use and Addiction
-
Table. Neurotransmitters Implicated in Drug Use and Addiction Neuro- transmitter Distribution in the Central Nervous System Functions
AffectedDrugs That
Affect ItDopamine - Midbrain
- Ventral Tegmental Area (VTA)
- Cerebral cortex
- Hypothalamus
- Pleasure and reward
- Movement
- Attention
- Memory
- Cocaine
- Methamphetamine
- Amphetamine
- In addition, virtually all drugs of abuse directly or indirectly augment dopamine in the reward pathway.
Serotonin - Midbrain
- VTA
- Cerebral cortex
- Hypothalamus
- Mood
- Sleep
- Sexual desire
- Appetite
- MDMA (ecstasy)
- LSD
- Cocaine
Norepinephrine - Midbrain
- VTA
- Cerebral cortex
- Hypothalamus
- Sensory processing
- Movement
- Sleep
- Mood
- Memory
- Anxiety
- Cocaine
- Methamphetamine
- Amphetamine
Endogenous opioids (endorphin and enkephalin) - Widely distributed in brain, but regions vary in type of receptors
- Spinal cord
- Analgesia
- Sedation
- Rate of bodily functions (e.g., breathing)
- Mood
- Heroin
- Morphine
- Prescription pain relievers (e.g., oxycodone)
Acetylcholine - Hippocampus
- Cerebral cortex
- Thalamus
- Basal ganglia
- Cerebellum
- Memory
- Arousal
- Attention
- Mood
- Nicotine
Endogenous cannabinoids (anandamide) - Cerebral cortex
- Hippocampus
- Thalamus
- Basal ganglia
- Movement
- Cognition and memory
- Marijuana
Glutamate - Widely distributed in brain
- Neuron activity (increased rate)
- Learning
- Cognition
- Memory
- Ketamine
- Phencyclidine
- Alcohol
Gamma-aminobutyric acid (GABA) - Widely distributed in brain
- Neuron activity (slowed)
- Anxiety
- Memory
- Anesthesia
- Sedatives
- Tranquilizers
- Alcohol
How Does the Drug Alter Neurotransmission?
As described above, neurotransmission is a cyclic process that transpires in several steps utilizing specialized components of the sending and receiving neurons. Identifying the precise step that a drug disrupts, and how, provides crucial insight into its impact on users, and is key to developing medical and behavioral interventions to inhibit, counter, or reverse the disruption.
Some drugs mimic neurotransmitters. Heroin and prescription opioids, for example, chemically resemble the brain’s natural opioids (endorphin and enkephalin) sufficiently to engage and stimulate their specialized receptors. Since heroin stimulates many more receptors more strongly than the natural opioids, the result is a massive amplification of opioid receptor activity. Marijuana mimics cannabinoid neurotransmitters, the most important of which is anandamide. Nicotine attaches to receptors for acetylcholine, the neurotransmitter for the cholinergic system.
Other drugs alter neurotransmission by interacting with molecular components of the sending and receiving process other than receptors. Cocaine, for example, attaches to the dopamine transporter, the molecular conduit that draws free-floating dopamine out of the synapse and back into the sending neuron. As long as cocaine occupies the transporter, dopamine cannot re-enter the neuron. It builds up in the synapse, stimulating receiving-neuron receptors more copiously and producing much greater dopamine impact on the receiving neurons than occurs naturally. The section “How Cocaine Motivates Drug Use and Causes Addiction” (below) enumerates some of cocaine’s interactions with the mechanisms of dopamine and other neurotransmitter signaling, and how they motivate use of the drug and contribute to dependence and addiction.
Finally, some drugs alter neurotransmission by means other than increasing or decreasing the quantity of receptors stimulated. Benzodiazepines, such as diazepam or lorazepam, produce relaxation by enhancing receiving neurons’ responses when the inhibitory neurotransmitter GABA attaches to their receptors.
What Changes Occur With Chronic Drug Use?
During the early phase of an individual’s drug experimentation, neurotransmission normalizes as intoxication wears off and the substance leaves the brain. Eventually, however, repeated drug use leads to changes in neuronal structure and function that cause long-lasting or permanent neurotransmission abnormalities. These alterations underlie drug tolerance (where higher doses of the drug are needed to produce the same effect), withdrawal, addiction, and other persistent consequences.
Some longer-term changes begin as adjustments to compensate for drug-induced increases in neurotransmitter signaling intensity. For example, the brain responds to repeated drug-induced massive dopamine surges in part by reducing its complement of dopamine receptors. This alleviates the drugs’ overstimulation of the dopamine system, but also contributes to features of drug dependence (e.g., susceptibility to drug withdrawal) and of addiction (e.g., compromised ability to respond to normal dopamine fluctuations produced by natural rewards). Similarly, methadone and some other opioids induce neurons to retract a portion of their mu opioid receptors, making them unavailable for further stimulation. The retraction is short-lived, after which the receptors return to the neuron surface, restoring normal responsiveness to subsequent stimulation. This dynamic of reducing and then restoring receptor availability may thwart the development of tolerance to these drugs. (Morphine, in contrast, does not cause receptors to retract, and the resulting opioid overstimulation triggers intracellular adjustments that appear to promote opioid tolerance.)
The drug-related mechanisms producing cumulative changes in neurotransmission sometimes are epigenetic in nature. While a drug cannot change a person’s genes, drugs can prod some genes to increase or decrease their production of proteins, leading to changes in neuron function or even actual reshaping of the physical structure of neurons. For example, in mice, cocaine alters important genetic transcription factors and the expression of hundreds of genes. Some of the resulting changes in the brain’s complement of proteins have been associated with increased drug-seeking and addiction-like behaviors in animals. Other changes, such as proliferation of new dendrites (branchlike structures on neurons that feature neurotransmitter receptors on their surface) may be compensatory. Some epigenetic changes can be passed down to the next generation, and one study found that the offspring of rats exposed to THC—the main psychotropic component of marijuana—have alterations in glutamate and cannabinoid receptor formation that affects their responses to heroin.
Some drugs are toxic to neurons, and the effect accumulates with repeated exposures. For example, the club drug methylenedioxymethamphetamine (MDMA [Ecstasy/Molly]) damages axons (the branch of a neuron that releases its neurotransmitter into the synapse) that release serotonin; the result is disruption of serotonin neurotransmission that may underlie the memory problems that are sometimes experienced by heavy users. Similarly, methamphetamine damage to dopamine-releasing neurons can cause significant defects in thinking and motor skills; with abstinence, dopamine function can partially recover, but the extent to which cognitive and motor capabilities can recover remains unclear.
Research Methods
Researchers employ a panoply of methods to investigate drugs’ effects on neurotransmitter systems, including brain tissue assays, live studies, brain scans, and genetic studies. To determine whether a drug affects a particular neurotransmitter system, or how, researchers typically will compare animals or people who have a history of drug exposure with others who do not. Researchers investigating whether a drug’s impact on neurotransmission underlies a drug-related behavior or symptom may compare neurotransmitter activity in animals or people who exhibit the behavior or symptom and others who do not. In experiments with animals, drug exposure often takes place under laboratory conditions designed to mimic human drug consumption. Studies can be divided into those in which measurements are made in living animals or people and those in which animal brain tissue is removed and examined.
Brain Tissue Assays
Scientists may perform chemical assays on brain tissue to quantify the presence of a neurotransmitter, receptor, or other structure of interest. In a recent experiment, scientists assayed brain tissue from 35-day-old rat pups and found that pups that had been exposed to nicotine in utero had fewer nicotinic acetylcholine receptors in the reward system than unexposed rats. The researchers speculated that if nicotine exposure has the same effect in humans, people whose mothers smoked while pregnant may require more puffs of a cigarette to obtain nicotine’s rewarding effect.
A second experimental method using brain tissue enables researchers to view a drug’s effects on neurotransmission in action. Scientists place the tissue in a laboratory solution of nutrients (cell culture) that enables neurons to survive outside of the body. The researchers may then, for example, add the drug being investigated to the solution and observe whether or not the neurons respond by increasing their release of neurotransmitters. Alternatively, they may measure neurons’ membrane or electrical properties that stimulate or inhibit neurotransmitter release or response.
In both living animals and extracted tissue, the techniques for measuring neurotransmitter quantities and fluctuations include microdialysis and fast-scan cyclic voltammetry (FSCV). Microdialysis involves taking a series of samples of the intercellular fluid containing the neurotransmitter through a microscopic tube inserted into the tissue or living brain. FSCV, which was developed by NIDA-funded scientists, monitors neurotransmitter fluctuations at tenth-of-a-second intervals by measuring electrical changes related to neurotransmitter concentrations.
Live Studies
Studies with living animals or people are essential for tying drugs’ effects on neurotransmitters to behaviors or symptoms. A common design for experiments with either animals or people is to give study subjects a chemical that has a known effect on a particular neurotransmitter, and then observe the impact on behavior. Typically, the chemical is either an agonist (promoter) or antagonist (blocker) of signaling by the neurotransmitter.
In one experiment, for example, researchers showed that glutamate levels fell when rats were withdrawn from cocaine after a period of self-administration, and restoring the animals’ glutamate levels with the medication acetylcysteine reduced their motivation to resume seeking the drug. Another team using a similar strategy showed that nicotine-induced disruption of glutamate signaling contributed to aspects of nicotine withdrawal. Both findings point to manipulation of the glutamate system as a potential strategy for treating some addictions.
A new research technique, optogenetics, enables researchers to raise and lower the activity of specific neurons or precisely targeted brain regions in living animals and observe the effects on the animals’ behavior. Using this technique, researchers shut down rats’ compulsive cocaine-seeking by increasing activity in the animals’ prelimbic cortex, a region that, like the human orbitofrontal cortex, contains many dopamine-responsive neurons and participates in value-based decision-making. Researchers are now attempting to parlay this discovery into a novel treatment for cocaine addiction.
Brain Scans
Brain imaging techniques enable neuroscientists to directly assess neurotransmission in people and living animals. With positron emission tomography (PET), researchers can compare people with and without a drug addiction, quantifying differences in their levels of a particular neurotransmitter (e.g., dopamine) or neurotransmission component (e.g., a receptor or transporter). One set of PET studies, for example, disclosed that smokers experienced cravings as long as nicotine occupied fewer than 95 percent of one type of receptor (α4β2*-nACh) in the brain, but that smoking nicotine-free cigarettes partially reduced craving. The findings indicated that the need to saturate these receptors is the primary driver of smoking behavior, but that sensory aspects of smoking, such as handling and tasting cigarettes, also play a role. With PET, researchers also can correlate a drug’s transit through the brain with fluctuations in a target neurotransmitter. Or, they can elicit a drug-related behavior or symptom (e.g., feeling high, craving) and correlate neurotransmitter fluctuations to the rise and fall in its intensity. (video)
Researchers use several imaging techniques, including PET, functional magnetic resonance imaging (fMRI), and computerized tomography to monitor metabolic activity in selected regions of the brain. Because each neurotransmitter has a unique distribution among the regions of the brain, information on locations of heightened or decreased activity provides clues as to which neurotransmitter is affected under the conditions of the study. Another technique, diffusion tensor imaging, provides information about the white matter (neuron fiber) pathways through which sending neurons extend to receiving neurons, often over long distances.
Genetic Studies
Studies that link genetic variants to contrasting responses to drugs and drug-related behaviors provide another avenue of insight into drugs and neurotransmission. Such studies have shown, for example, that one rare variant of the gene for the mu opioid receptor is twice as common in the general population of European Americans as it is among European Americans who are addicted to cocaine or opioids. The finding suggests that receptors that are built based on the DNA sequence of the variant gene confer resistance to those addictions. Another study linked a different variant of the same mu opioid receptor gene to reduced incidence and severity of neonatal abstinence syndrome among infants born to mothers who used opioids while pregnant. Medicinal chemists may be able to use such information to design new pain relievers that are as effective as opioids but avoid opioids’ undesirable effects. Eventually, doctors may be able to use a patient’s genetic information to guide their pain treatment strategies, weighing patients’ genetic risks for addiction when deciding whether to use opioids to treat their pain.
In another type of study, researchers knock down or knock out specific genes in laboratory animals and observe whether drug-related behavior—for example, pacing restlessly after being given a stimulant—increases or decreases. Researchers have used this technique to explore how different subtypes of nicotinic acetylcholine receptor influence smoking behaviors, including how much a person smokes and susceptibility to symptoms of nicotine withdrawal.
Finally, researchers may implant modified genes into animals. In one such project, researchers, starting from clues provided by a South American caterpillar that eats coca leaves, modified the dopamine transporter gene to produce a transporter that is insensitive to cocaine. Mice who were implanted with this gene showed no preference for the drug over a saline solution. This result could point researchers toward medications capable of preventing or treating cocaine use disorders.
How Cocaine Motivates Drug Use and Causes Addiction
Research on cocaine illustrates how a drug can disrupt neurotransmission in multiple ways to promote intensified drug use, dependence, and addiction. Like all drugs that cause dependence and addiction, cocaine alters dopamine signaling. Studies, mostly with animals, indicate that the interactions of cocaine with the dopamine and other neurotransmitter systems influence the risk of drug use, progression to addiction, and relapse after abstinence through a variety of pathways.
Reward
- Cocaine causes pleasurable feelings that motivate drug use by sharply elevating dopamine concentrations in the synapses of the reward system
- Cocaine raises synaptic dopamine levels by preventing dopamine transporters from removing dopamine from the synapse and by stimulating dopamine-releasing neurons to release dopamine that they normally hold in reserve.
- Cocaine-induced increases in dopamine signaling promote repeated cocaine use by increasing the activity of dopamine type D1 receptors in a circuit that supports the conversion of urges into action, while suppressing the activity of dopamine type D2 receptors in an opposing circuit, and by increasing the activity of dopamine type D3 receptors.
- An animal’s higher social position or exposure to a stimulating environment may limit cocaine’s power to motivate repeated use by increasing the activity of dopamine D2 receptors.
Transition to Addiction
- Cocaine sensitizes dopamine-releasing neurons in the reward system, such that repeated exposures trigger the release of increasing amounts of dopamine, potentially ratcheting up urges to use the drug again.
- The increase in dopamine-releasing neurons’ responsiveness to cocaine begins with the first exposure to the drug and even occurs with only modest doses.
- The enhanced urge for the drug with diminished control over the urge that occurs briefly following cocaine use may become an abiding state, as repeated exposure to the drug prolongs the activity imbalance in favor of urge-promoting dopamine type 1 neurons over inhibitory dopamine type 2 neurons (see also here).
- With repeated cocaine exposure, some glutamate receptors in the reward system become sensitized to cocaine cues, programming the brain to assign primary importance to reacting to the cues.
- Cocaine appears to limit the brain’s ability to alter neurotransmission pathways in response to new experiences, which potentially limits a user’s ability to develop new behavioral alternatives to drug taking.
Craving and Relapse
- Cocaine craving builds in intensity during early abstinence, linked to the proliferation of a rare type of glutamate receptor.
- Even after extended abstinence, encounters with drug cues (i.e., things in the environment that are associated with previous drug experiences) cause dopamine to tick up in the reward system, and can rekindle powerful urges to take the drug.
- Mu opioid receptors in the frontal and temporal regions of the cortex appear to affect the intensity of a person’s craving for cocaine during his or her first few months of abstinence from the drug.
- Stress increases the likelihood that a former cocaine user’s single lapse will turn into an extended relapse because the stress hormone corticosterone augments the dopamine surge caused by cocaine
Summary
By altering neurotransmission, drugs can produce effects that make people want to use them repeatedly and induce health problems that can be long lasting and profound. Some important effects are shared by all drugs that cause dependence and addiction, most prominently disruption of the dopamine neurotransmitter system that results in initial pleasurable feelings and, with repeated use, potential functional and structural changes to neurons. There are also drug-specific effects: Each drug disrupts particular neurotransmitters in particular ways, and some have toxic effects on specific types of neurons.
Scientists use a wide variety of experimental tools and techniques to study drugs' effects on neurotransmission, and their consequences, in both animals and people. Their findings enhance our understanding of the experiences of drug users and the plight of people who are addicted, point the way to new behavioral and medication treatments, and provide potential bases for prevention strategies and monitoring progress in treatment.
- Appendix 1 - Drug Effects on Neurotransmission and the Phenomena of Dependence and Addiction
-
Appendix 1. Drug Effects on Neurotransmission and the Phenomena of Dependence and Addiction Abused Drug Neurotransmission Element Observation Interpretation NIDA Notes Articles "Bath Salts" Dopamine and serotonin transporters (finding in rats) The psychoactive ingredient in bath salts caused these transporters to reverse their activity, releasing the neurotransmitters into the extracellular space rather than drawing them into the intracellular space. Heightened extracellular levels of both neurotransmitters underlie the drugs’ rewarding properties and other effects. Stimulants in “bath salts” produce pharmacological effects similar to MDMA Benzodiazepines (Valium®, Xanax®, etc.) Alpha-1 gamma-aminobutyric acid type A (GABAA) receptors (finding in mice) Mice that were genetically altered to prevent benzodiazepines from stimulating alpha-1 GABAA receptors did not develop dopamine surges or exhibit a preference for drug over sugar water. Benzodiazepines’ rewarding effects are caused by dopamine surges that in turn are caused by the drugs’ stimulation of alpha-1 GABAA receptors. Well-known mechanism underlies benzodiazepines' addictive properties Cocaine Dopamine-releasing cells (finding in mice) Cocaine precipitates dopamine release from a reserve pool of intracellular vesicles. The additional dopamine release exacerbates dopamine surges that underlie cocaine’s rewarding and addictive effects. Cocaine can mobilize stored dopamine Dopamine transporter (finding in mice) Animals with a genetic manipulation that desensitized the dopamine transporters to cocaine did not prefer the drug over saline. Medications that selectively desensitize the dopamine transporter to cocaine may prevent relapse. Mice with genetic alteration eschew cocaine Dopamine type 2 receptors (finding in monkeys) In monkeys, cocaine dose relatedly suppressed availability of the receptors, and monkeys with fewer receptors self-administered more cocaine. People who naturally have fewer of the receptors may be more sensitive to cocaine reward and, therefore, more vulnerable to cocaine abuse and addiction. Low dopamine receptor availability may promote cocaine addiction Dopamine receptors (finding in mice) A cocaine-induced imbalance between two types of dopamine receptors lasted only briefly following a first dose of the drug, but was prolonged following later doses. The prolongation of this imbalance may underlie the transition from voluntary to compulsive cocaine use. Why do people lose control over their cocaine use? Delta opioid receptors (DORs) (finding in rats) Animals pretreated with a test compound that stimulates DORs exhibited decreased signs of anxiety and depression when withdrawn from cocaine. Medications that stimulate the DOR may ease anxiety and depression when people are withdrawn from cocaine. Test substance attenuates signs of cocaine withdrawal in rats Mu opioid receptor (MOR) Study participants whose MOR levels in the frontal and temporal cortex fell less during 3 months of abstinence relapsed sooner than those whose levels fell more. MORs in these brain areas may contribute to drug craving during abstinence. Brain opioid levels predict time to cocaine relapse Glutamate AMPA receptors (finding in rats) Cocaine exposure heightened glutamate AMPA receptors’ responsiveness to cocaine-associated cues. Cocaine users’ susceptibility to cocaine-associated cues may in part reflect drug-induced heightening of glutamate signaling via AMPA receptors. New insight into how cues cause relapse to cocaine Organic cation transporter 3 (OCT3) (finding in rats) By blocking OCT3, the stress hormone corticosterone enhances cocaine-induced dopamine surges in rats. The association between OCT3 and dopamine may explain why stress is a potent relapse trigger. Stress hormone sets the stage for relapse to cocaine use Cocaine, Heroin Mu opioid receptor type M1 (OPRM1) A variant of the OPRM1 gene is more common among non-addicted people than among people who are addicted to heroin or cocaine. The OPRM1 variant, which renders the mu opioid receptor less responsive to some opioids, may reduce the risk of addiction to heroin or cocaine. Gene variants reduce opioid risks Heroin OPRM1 Among newborns who were prenatally exposed to methadone or buprenorphine, those with a variant of the OPRM1 gene had reduced severity of neonatal abstinence syndrome. The OPRM1 variant is protective against neonatal abstinence syndrome. Gene variants reduce opioid risks Nicotine Dopamine levels in the brain’s reward system Smokers’ self-reports of mood elevation while smoking correlated with dopamine levels in their reward system. Nicotine enhances smokers’ mood by increasing dopamine in the reward system. Nicotine boosts mood, brain dopamine levels Nicotinic acetylcholine receptors (nAChRs) of the α4β2* subtype Smokers were found to crave nicotine whenever nicotine occupied fewer than 95 percent of their α4β2*-nAChRs. Smoking behaviors are driven partly by the need to keep α4β2*-nAChRs saturated. Imaging studies elucidate neurobiology of cigarette craving nAChRs that contain the α5 subunit (α5*-nAChRs) (findings in mice) Mice bred to lack α5*-nAChRs self-administered more nicotine than normal mice. Nicotine stimulation of α5*-nAChRs in the habenula promotes aversive responses to the drug. In animals, receptor puts brakes on nicotine consumption nAChRs that contain the β2 subunit (β2*-nAChRs) Males who recently quit smoking had higher β2*-nAChR availability than males who never smoked, and females who recently quit smoking had lower β2*-nAChR availability than females who never smoked. Men’s and women’s β2*-nAChRs respond differently to nicotine, possibly explaining some gender differences in smoking behaviors and responses to treatment for nicotine addiction. Receptor may underlie gender differences in response to smoking cessation nAChRs that contain the β2 subunit (β2*-nAChRs) The deficit in β2*-nAChR availability among some smokers who recently quit relative to never-smokers, took more than a month to resolve. A smoking-induced deficit in β2*-nAChR availability may account for some of the lethargy, moodiness, and confused thinking people experience after quitting cigarettes. Abstinent smokers’ nicotinic receptors take more than a month to normalize Toluene Dopaminergic (dopamine-releasing) neurons (finding in rats) Blocking dopaminergic neurons’ release of the neurotransmitter reduced, and increasing dopamine release increased, toluene-induced locomotor activity. Toluene causes locomotor stimulation through stimulating neurons to release dopamine into the reward system. Dopamine enhancement underlies a toluene behavioral effect
- Appendix 2 - Medications and Interventions for Drug Dependence That Alter Neurotransmission
-
B: Investigational Medications and Interventions Abused Drug Treatment Site of Potential Therapeutic Action Observation Potential Therapeutic Use NIDA Notes Article Cannabis, Cocaine, Heroin Acetylcysteine Glutamate transporters on glial cells Accelerated rats’ extinction learning and reduced animals’ resumption of heroin seeking Facilitate learning in behavioral therapy and prevent relapse following abstinence A potential medication for marijuana dependence
N-acetylcysteine postsynaptic effect limits efficacy
Medications that normalize brain glutamate reduce drug seeking in ratsCocaine Long-acting mixed amphetamine salts Dopaminergic (dopamine-releasing) and noradrenergic (norepinephrine-releasing) neurons Reduced both cocaine use and ADHD symptoms in patients with both problems Support abstinence from cocaine use in patients who also have ADHD Slow-release amphetamine medication benefits patients with comorbid cocaine addiction and ADHD Naltrexone Mu opioid receptors In combination with disulfiram, increased abstinence from cocaine and alcohol in patients addicted to both substances Support abstinence from cocaine and alcohol use in patients who have both addictions Combined treatments improve dual abstinence Transcranial magnetic stimulation Dopaminergic (dopamine-releasing) neurons Reduced patients’ cocaine use and craving Ease the transition to abstinence and prevent relapse Can magnets treat cocaine addiction? Heroin and Prescription Opioids Lofexidine Norepinephrine receptors Reduced stress-induced and cue-induced relapse to opioid use among patients being treated with naltrexone Ease withdrawal and prevent relapse Lofexidine may enhance naltrexone efficacy Memantine Glutamate receptors Reduced patients’ heroin use and craving following short-term treatment with buprenorphine/naloxone Ease the transition to abstinence and prevent relapse Dual regimen aims to shorten medication-assisted therapy